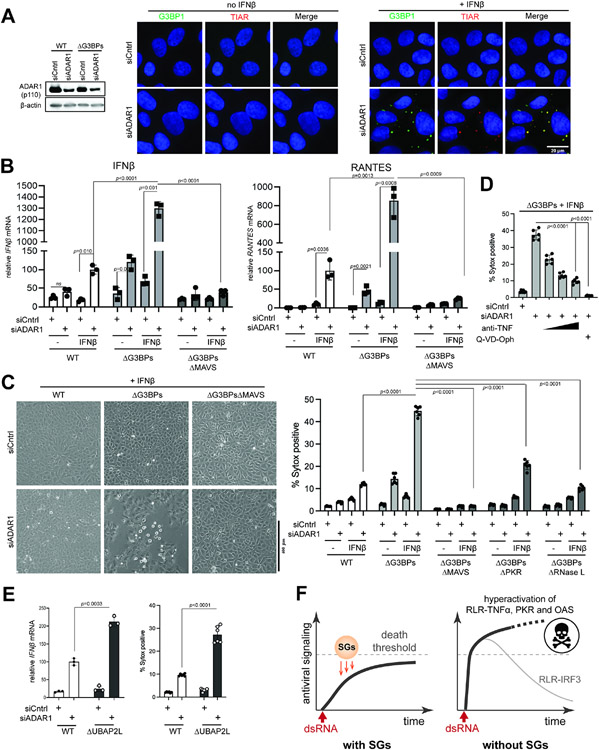
Figure 7. SGs suppress immune response to self-derived dsRNAs under the ADAR1 deficiency.
A. IF analysis of G3BP1 (green) and TIAR (red) in U2OS cells in the presence or absence of ADAR1 knock-down and IFNβ priming. Cells were transfected with siRNA for 24 hrs and then treated with IFNβ (10 ng/ml) for additional 24 hrs prior to imaging.
B. Antiviral signaling in U2OS cells upon ADAR1 knock-down. Data were normalized to WT cells in the presence of IFNβ priming and ADAR1 knock-down.
C. Cell death upon ADAR1 knock-down, as measured by brightfield images (left) and Sytox uptake (right).
D. Effect of anti-TNF and pan-caspase inhibitor (Q-VD-Oph) on cell death upon ADAR1 knock-down.
E. Antiviral signaling and cell death upon ADAR1 knock-down in U2OS cells. All samples were treated with IFNβ (10 ng/ml). Data were normalized to WT cells in the presence of ADAR1 knock-down.
F. Schematic summarizing the roles of SGs in protecting cells from dsRNA. SGs suppress a broad range of dsRNA-triggered innate immune pathways (RLR, PKR and OASes), regardless of the origin of dsRNA. In particular, SGs slow down the ramp-up speed of RLR signaling and help maintain its magnitude below the “death” threshold. In the absence of SGs, RLRs are hyperactivated, leading to an excessive innate immune response and consequent cell death. The IRF3-IFN axis downstream of RLR-MAVS does not contribute to cell death and often displays a dynamic temporal behavior characterized by a sharp peak followed by a strong decline due to caspase-dependent feedback regulation.
Data are presented in means ± SD. p values were calculated using two-tailed unpaired Student’s t test (ns, p>0.05). All data are representative of three independent experiments.