Figure 6.
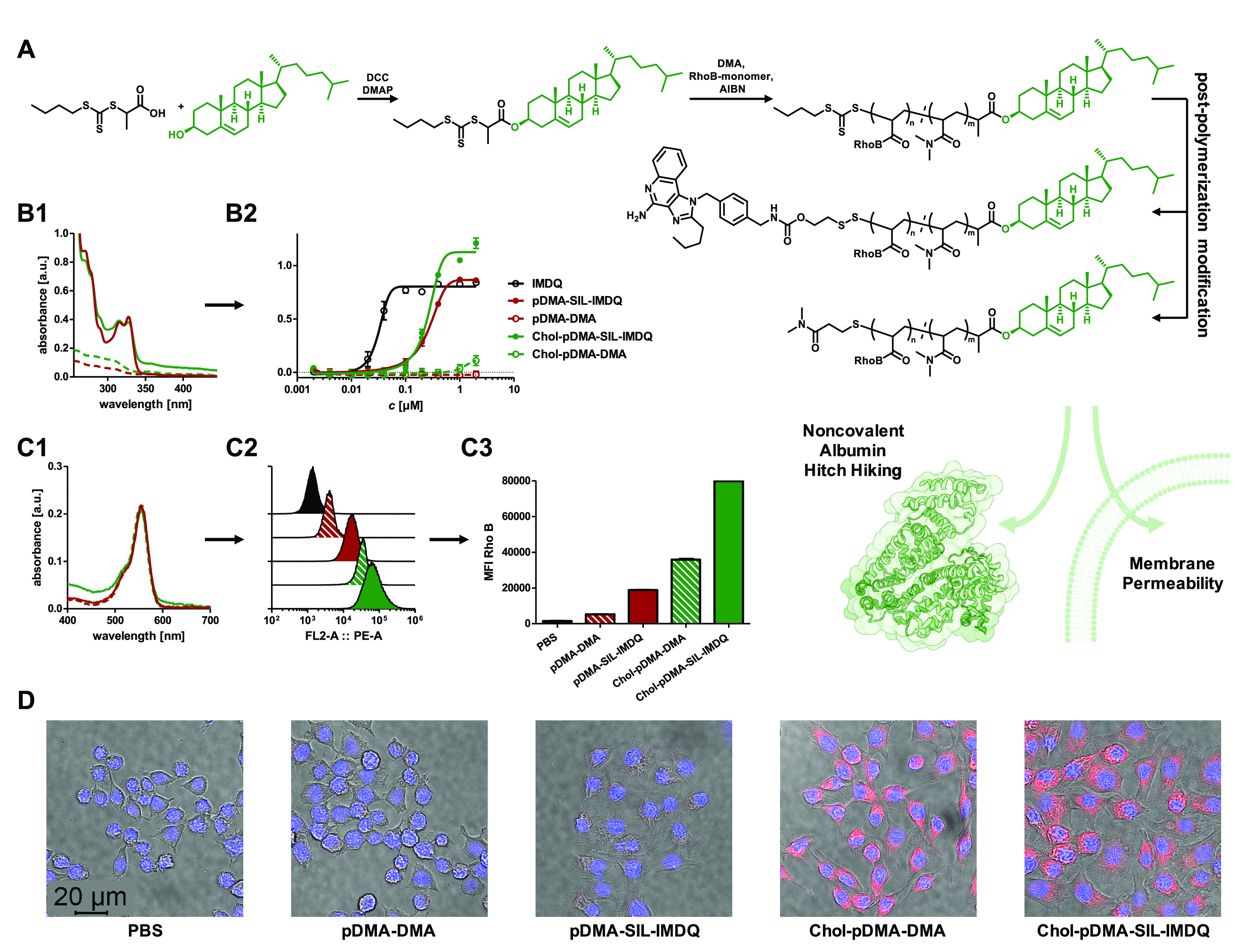
Comparison of RAFT polymer-derived SIL–immunodrug conjugates with and without α-end cholesterol modification. (A) Synthetic scheme of chain-transfer agent modification with cholesterol prior to polymerization. Resulting polymer-bearing cholesterol moiety exhibiting improved membrane permeability and capability of noncovalently binding to albumin. The trithiocarbonate end group can still be exploited for SIL–immunodrug conjugation (targeted average DP = 40). (B) Successful immunodrug conjugation verified by characteristic IMDQ absorbance at 322 nm via UV–vis spectroscopy confirming similar loading for polymer–drug conjugates (B1) prior to their application on RAW-Blue macrophages and subsequent QUANTI-Blue reporter cell assay readout. The recorded absorbances reveal a similar level of IMDQ activity at given concentrations for the polymers with and without cholesterol modification. Interestingly, the cholesterol IMDQ conjugate provided a more intense activity; however, cholesterol functionalization itself also exhibited a slight intrinsic immune activation (B2). The cholesterol polymer conjugate without IMDQ also slightly triggers immune activation at these elevated concentrations. (C) Polymer samples with similar fluorescent labeling determined by UV–vis spectroscopy (C1) were applied on RAW-Blue macrophages leading to the respective histograms by flow cytometry (C2). Uptake was generally found at higher levels for the cholesterol-functionalized polymers, which could further be quantified by mean fluorescence intensity analyses (C3). (D) Merged confocal microscopy images confirming enhanced cellular uptake and internalization for the cholesterol-modified fluorescently labeled polymers (1BM0 was used for albumin structure and processed with BioRender.com).
