Table 1.
Details of substitutions and anti-HIV activity for N-[4-(4’-chlorophenyl) thiazol-2-yl] thiosemicarbazide (3a–l, Fig. 7)
| Compound No. | R | R1 | EC50 (μg)a |
|---|---|---|---|
| 3a | H | H | >12 |
| 3b | Cl | H | >10 |
| 3c | Br | H | >10 |
| 3d | H | -CH2-N(CH3)2 | >54 |
| 3e | H | 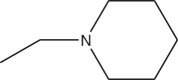 |
>57 |
| 3f | H | 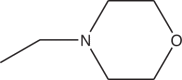 |
>19 |
| 3g | Cl | -CH2-N(CH3)2 | >10 |
| 3h | Cl | 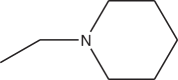 |
>12 |
| 3i | Cl | 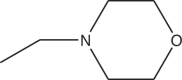 |
>12 |
| 3j | Br | -CH2-N(CH3)2 | >29 |
| 3k | Br | 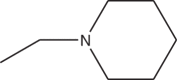 |
>21 |
| 3l | Br | 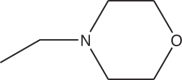 |
>14 |
aMT-4 cells are 50% protected by an effective concentration of the chemical from HIV’s cytotoxic effects
