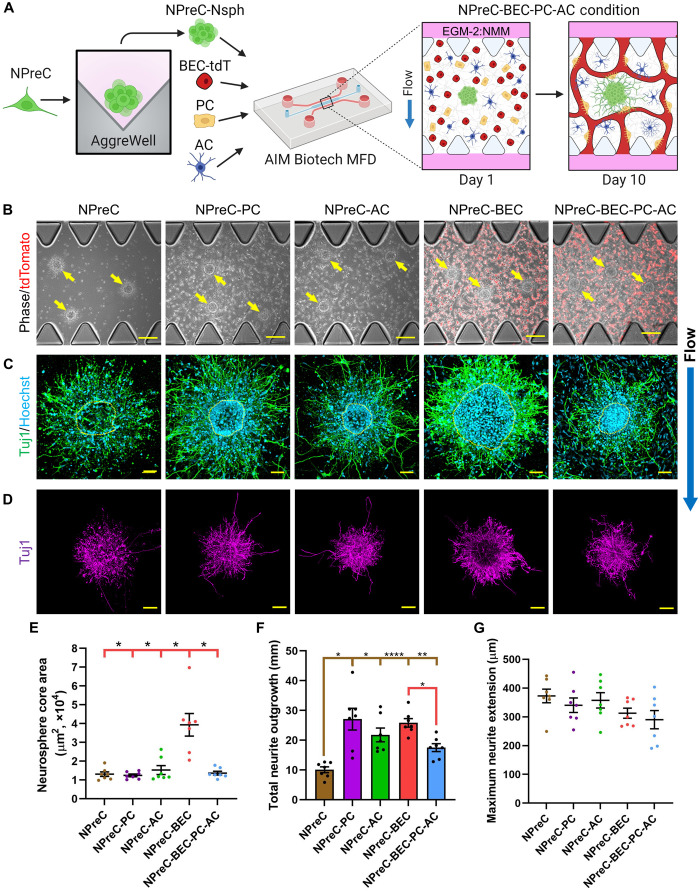
Fig. 5. NPreC neurosphere (NPreC-Nsph) neurogenesis in MFDs.
(A) Illustration of the culture protocol used to investigate NPreC-Nsph neurogenesis. Briefly, NPreC-Nsphs were cultured alone (NPreC condition), with PCs (NPreC-PC condition), with ACs (NPreC-AC condition), with BECs-tdT (NPreC-BEC condition), and with all three cell types (NPreC-BEC-PC-AC condition) in fibrin gels within MFDs. Samples were cultured for 10 days in EGM-2:NMM under flow conditions. Illustration only shows NPreC-BEC-PC-AC condition. (B) Phase/fluorescence images of NPreC-Nsphs in all conditions on day 1. Yellow arrows identify NPreC-Nsphs. BECs-tdT expressed tdTomato (red). (C) Fluorescence confocal MIPs of NPreC-Nsphs in all conditions on day 10. NPC-neurons were labeled with Tuj1 (green) and Hoechst (blue). BECs-tdT, PCs, and ACs are not shown. Yellow dotted lines outline neurosphere cores. (D) Large fluorescence confocal MIPs of NPreC-Nsphs (Tuj1, purple) in all conditions. Background signals were removed to highlight neurites. Scale bars, 200 μm (B), 50 μm (C), and 100 μm (D). Blue arrow indicates direction of IF. (E to G) Graphs showing the neurosphere core area (E), total neurite outgrowth (F), and maximum neurite extension (G) measured for NPreC-Nsphs from all conditions on day 10. The data show mean value, error bars ± SEM, data from N = 7 neurospheres from n = 3 MFDs, Welch’s ANOVA with Dunnett’s test, the absence of significance line indicates P > 0.05, *P < 0.05, **P < 0.01, ****P < 0.0001. Brown and red significance lines show statistical comparisons made for NPreC and NPreC-BEC conditions, respectively.