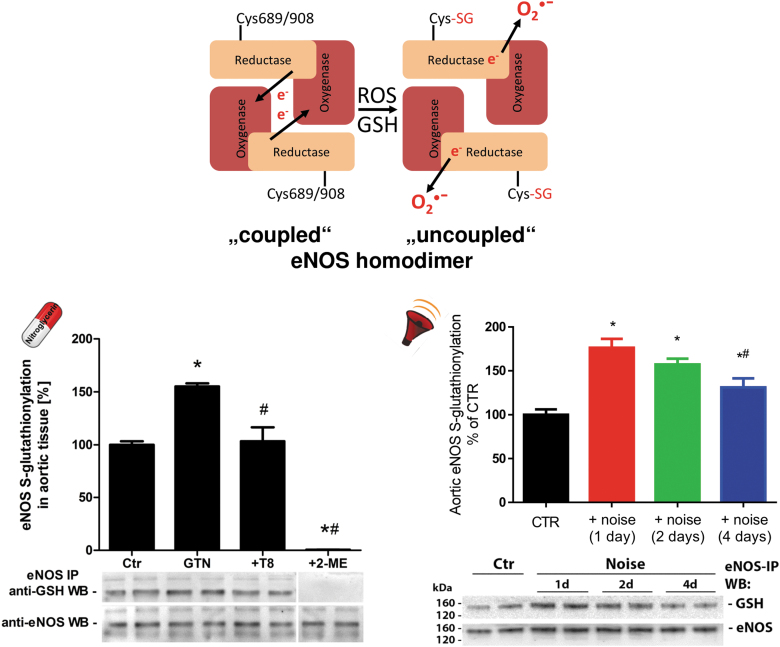
FIG. 3.
S-glutathionylation of eNOS in GTN-treated nitrate-tolerant rats and aircraft noise-exposed mice. Upper part: Oxidative stress causes S-glutathionylation of eNOS at cysteine 689 and 908, a surrogate marker for uncoupling of the protein. In the “coupled” eNOS homodimer, electrons are usually transferred from the NADPH and flavins to the heme iron. When cysteine residues 689 and/or 908 undergo S-glutathionylation, structural changes in eNOS are initiated that are followed by misdirection of the electrons to molecular oxygen with subsequent •O2− formation, termed “uncoupled” state of eNOS. Lower part: S-glutathionylation of eNOS was determined by eNOS immunoprecipitation from aortic tissue, followed by antiglutathione staining and normalization on eNOS. S-glutathionylation of eNOS was increased in nitrate-tolerant rats (GTN with a dose of 50 mg/kg/day for 3.5 days), which was normalized by telmisartan cotherapy (T8, 8 mg/kg/day for 10 days) (Knorr et al, 2011). *p < 0.05 versus Ctr, #p < 0.05 versus GTN group. S-glutathionylation of eNOS was also increased by aircraft noise exposure [72 dB(A) around-the-clock for 1, 2, or 4 days] (Munzel et al, 2017a). Disappearance of the antiglutathione staining in the presence of 2-mercaptoethanol served as a control. *p < 0.05 versus Ctr, #p < 0.05 versus noise (1 day) group. Representative blots are shown at the bottom of each densitometric quantification along with the respective loading control. Scheme in the upper part was adapted from Daiber et al (2020b) with permission. Data in the lower part were reproduced from Knorr et al (2011) (for nitrate tolerance) and Munzel et al (2017a) (for noise exposure) with permission.