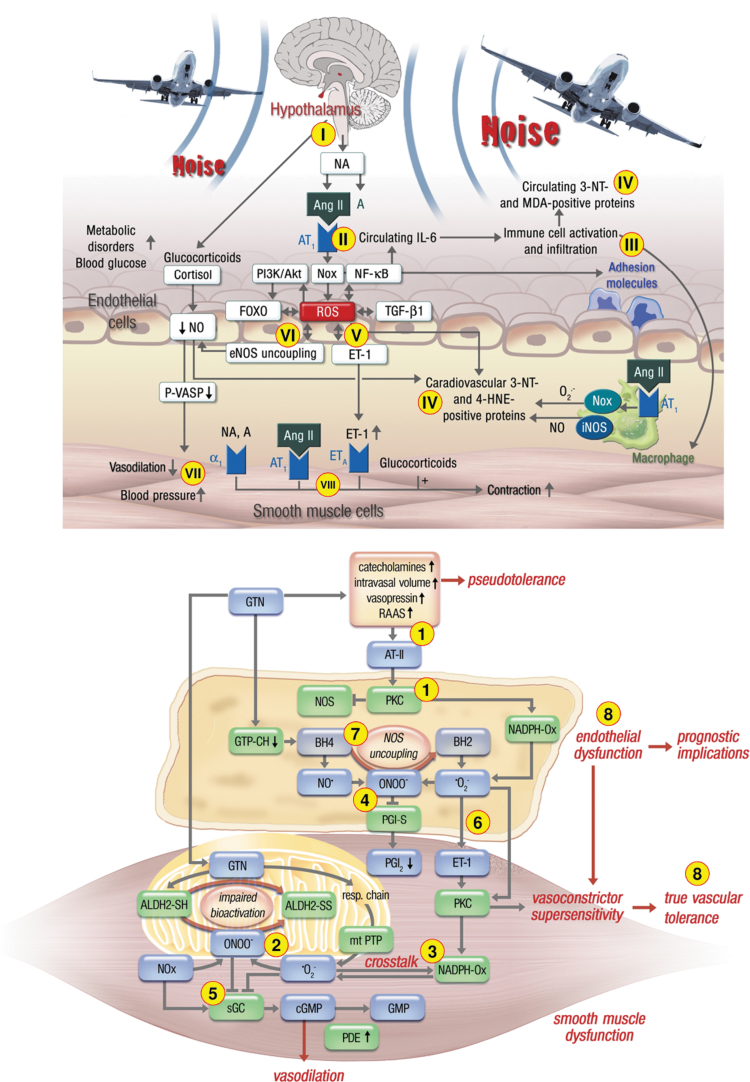
FIG. 6.
Summarizing schemes on the similarities and differences of pathomechanisms underlying nitrate tolerance (upper part) and transportation noise exposure (lower part)-mediated cardiovascular damage. (1) Chronic GTN therapy leads to activation of the RAAS via nitrovasodilator-induced hypotension with subsequent angiotensin-II release, PKC and NOX-2 activation (also called pseudotolerance). In addition, GTN therapy causes substantial mtROS formation also causing inhibition of ALDH-2, the GTN-bioactivating enzyme (2), followed by redox-dependent NOX-2 activation (3) and peroxynitrite (ONOO−) formation with subsequent dysregulation of other critical regulators of vascular tone by nitration of prostacyclin synthase (PGI-S) (4), oxidation of sGC (5), oxidative induction of ET-1 (6), and uncoupling of eNOS by BH4 depletion and S-glutathionylation (7), all of which leads to endothelial dysfunction and true vascular tolerance (8). In contrast, traffic noise exposure causes neuronal stress responses via the hypothalamic–pituitary–adrenal axis (release of cortisol/corticosterone) and the sympathetic nervous system (release of NA) (I) with subsequent RAAS and NOX-2 activation (II) along with enhanced inflammation (III). The resulting oxidative stress leads to peroxynitrite formation, protein tyrosine nitration, and lipid peroxidation (IV), as well as oxidative induction of ET-1 (V) and eNOS uncoupling (VI), all of which lead to endothelial dysfunction and hypertension (VII), in part, mediated by direct vasoconstrictor effects (VIII). The major differences of the pathomechanisms of nitrate tolerance and noise exposure are the pathways that lead to activation of the RAAS, the substantial adverse effects of GTN on mitochondrial function and mtROS formation, and the strong inflammatory component underlying noise exposure-mediated damage. The major similarities are the central role of oxidative stress in both pathomechanisms of endothelial dysfunction, with NOX-2, RAAS, and eNOS uncoupling as key players. The schemes were reused from Munzel et al (2011) (upper part) and Munzel et al (2017a) (lower part) with permission. ALDH-2, mitochondrial aldehyde dehydrogenase; ET-1, endothelin-1; mtROS, mitochondrial reactive oxygen species; NA, noradrenalin; RAAS, renin–angiotensin–aldosterone system; sGC, soluble guanylyl cyclase.