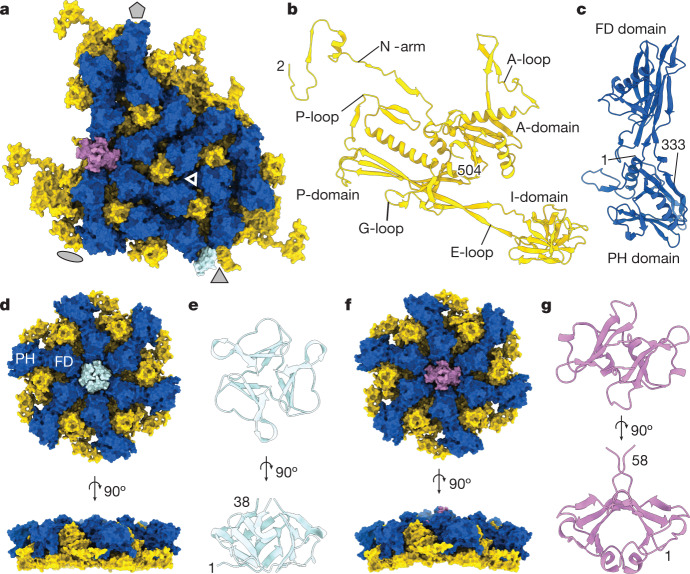
Fig. 2. ΦcrAss001 capsid proteins.
a, Molecular surface of the asymmetric subunit of the icosahedral capsid with indicated symmetry axes (grey shapes), with major capsid protein gp32 (yellow), capsid auxiliary protein gp36 (dark blue), head fibre proteins gp21 (HFT; pale blue, trimer) and gp29 (HFD; pink, dimer). The triangle indicates a local quasi-C3 symmetry formed by I and PH domains. b,c, Ribbon diagrams of the major capsid protein subunit (b) and the capsid auxiliary protein gp32 (c). d, Molecular surface of the C3 capsid hexon. e, Ribbon diagram of the HFT trimer. f, Molecular surface of the skewed hexon. g, Ribbon diagram of the HFD dimer. Numbers indicate terminal residues of the model.