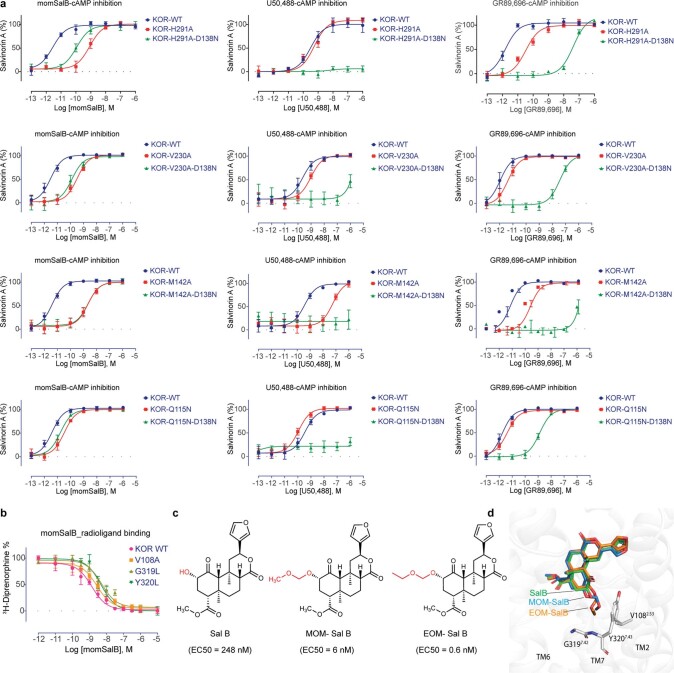
Extended Data Fig. 3. Molecular determinants of momSalB agonism.
a. The positive effect of additional D1383.32N mutation on the momSalB-mediated cAMP inhibition through KOR. The additional D1383.32N mutation does not rescue U50,488 or GR89,696-mediated cAMP inhibition. Data are grouped data ± s.e.m. from n = 3 biological replicates. Full quantitative parameters from this experiment are listed in Supplementary Table 10. b. Effects of mutations in the hydrophobic pocket on the binding affinity of momSalB. Data are grouped data ± s.e.m. from n = 3 independent biological replicates. Full quantitative parameters from this experiment are listed in Supplementary Table 11. c and d. Chemical structures of SalB, momSalB, and EOM-SalB. Differences are highlighted by red colour. The agonist activity of each analogue is shown in the parentheses. Data for EOM-SalB was taken from ref. 19. Binding poses of SalB and EOM-SalB at KOR were revealed by molecular docking performed in the Schrodinger Maestro v12.9. The three ligands occupy a similar binding pocket with different extents toward the hydrophobic pocket.