Abstract
The prevalence of mental health disorders is high among people with Cystic Fibrosis. The psychological symptoms in CF are associated with poor adherence, worse treatment outcomes, and greater health utilization/cost. Mental health and neurocognitive Adverse Events (AEs) have been reported with all available Cystic Fibrosis Transmembrane conductance Regulator (CFTR) modulators in small groups of patients. We report our experience with a dose reduction strategy in 10 of our patients on elexacaftor/tezacaftor/ivacaftor (7.9% of total number of patients) who self-reported developing intense anxiety, irritability, sleep disturbance and/or mental slowness after initiation of full dose treatment. Standard dose elexacaftor/tezacaftor/ivacaftor resulted in 14.3 points improvement in mean Percent Predicted Forced Expiratory Volume in 1 s (ppFEV1), and a mean difference in sweat chloride of −39.3 mmol/L. We initially discontinued and/or reduced therapy according to the AEs severity, with a subsequent planned dose escalation every 4–6 weeks guided by sustainability of clinical effectiveness, absence of AEs recurrence, and patients’ preferences. Clinical parameters including lung function and sweat chloride were monitored for up to 12 weeks to assess ongoing clinical response to the reduced dose regimen. Dose reduction resulted in resolution of self-reported mental/psychological AEs, without loss of clinical effectiveness (ppFEV1 was 80.7% on standard dose, and 83.4% at 12 weeks on reduced dose; sweat chloride was 33.4 and 34 mmol/L on standard and reduced dose, respectively). Furthermore, in a subgroup of patients who completed 24 weeks of the reduced dose regimen, repeat low dose Computed Tomography imaging showed a significant response when compared to pre-initiation of elexacaftor/tezacaftor/ivacaftor.
Keywords: case report, cystic fibrosis transmembrane conductance regulator, CFTR, anxiety, FEV-1, sweat chloride, elexacaftor/tezacaftor/ivacaftor, side-effects
1 Introduction
The prevalence of mental health disorders is high among people with Cystic Fibrosis (CF) (Quittner et al., 2014; Quittner et al., 2016). It is estimated that 5%–19% of CF adolescents and 13%–29% of CF adults have depression (Latchford and Duff, 2013; Quittner et al., 2014), along with 22% of CF adolescents and 32% of CF adults experiencing anxiety (Quittner et al., 2014). The psychological symptoms in CF have been linked to poor treatment adherence (Hilliard et al., 2015), worse clinical outcomes (Snell et al., 2014; Quittner et al., 2016), and greater healthcare utilization and costs (Snell et al., 2014).
Over the last decade, treatment with Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) modulators has resulted in significant clinical benefits. More recently, a triple combination CFTR modulator using the next-generation corrector Elexacaftor in combination with Tezacaftor/Ivacaftor (ETI), has shown high clinical efficacy in patients homozygous or heterozygous for Phe508del mutation (Heijerman et al., 2019; Middleton et al., 2019; Barry et al., 2021). Despite the positive clinical outcomes for the majority of patients, mental health and neurocognitive Adverse Events (AEs) have been reported in real-world studies with all available CFTR modulators among small groups of patients (McKinzie et al., 2017; Talwalkar et al., 2017; Dagenais et al., 2020; Heo et al., 2022). The recently published Phase 3b clinical trial of ETI showed that one participant (1/87) in the ETI group discontinued treatment due to an adverse AE of anxiety and depression, and two participants (2/88) in the tezacaftor/ivacaftor group discontinued treatment due to AEs of psychotic disorder and obsessive-compulsive disorder (Sutharsan et al., 2022). In addition, a recently published real-world study showed an incidence of self-reported mental AEs in 7.1% of patients on ETI treatment (Spoletini et al., 2022).
The mechanism behind mental health AEs reported during treatment with CFTR modulators has not been fully illuminated, but the potential pathways include: 1) Drug-drug interaction between CFTR modulators and psychotropic medications through cytochrome P450 (Jordan et al., 2016; McKinzie et al., 2017). CFTR modulators, specifically ivacaftor and lumacaftor may affect the activities of cytochrome P450 isoenzymes (CYP2C9, CYPC19, CYP3A4), therefore this may alter the level of other cytochrome P450 substrates including selective serotonin reuptake inhibitors (SSRIs) and benzodiazepines. 2) The direct effect of CFTR modulators and their metabolites on the serotonin receptors and the CFTR receptors that are expressed ubiquitously in the nervous system (Marcorelles et al., 2014; Schneider et al., 2018).
The potential mental health AEs of CFTR modulator therapy, have focused our attention on the Phase 2 clinical trial (Keating et al., 2018). This trial demonstrated a variable but clinically significant response to different doses of the triple CFTR modulator ETI. Doses as low as 50 mg of VX-445 (elexacaftor) resulted in improvements in Percent Predicted Forced Expiratory Volume in 1 s (ppFEV1) and sweat chloride of almost 11% and −38 mmol/L respectively (Keating et al., 2018). In addition, the potential clinical effectiveness of a lower dose strategy based on this Phase 2 study, allowed us to develop an adaptive strategy where we adopted a dose reduction protocol in the subgroup of patients with self-reported intense anxiety, irritability, sleep disturbance and/or mental slowness, in an attempt to minimize AEs while continuing CFTR modulator therapy. We report our experience with a dose reduction strategy.
2 Case series description
2.1 Response to full standard dose of ETI
As a standard practice in our institution, along with standard clinical measures, all adult CF patients are screened for anxiety and depression prior to commencing ETI treatment (baseline) via a series of established questionnaires, specifically the Hospital Anxiety and Depression Scale (HADS), the Patient Health Questionnaire (PHQ-9), and General Anxiety Disorder-7 (GAD-7).
Between October 2020 and May 2021, we initiated ETI with 126 adult CF patients attending our service. Subsequently, a total of 10 patients (7.9% of patients on ETI) with no known psychological disorders or history of strong cytochrome P450 inducer or inhibitor use, developed self-reported anxiety, irritability, sleep disturbance and/or mental slowness within four weeks of initiation of full-dose treatment. None of these patients reported any anxiety or depression prior to ETI, and their baseline depression and anxiety screening questionnaires were within normal. The average weight and BMI of this group of patients at the start of ETI therapy were 68 kg (SD 13.9, min–max 48.7–86.4 kg) and 23.7 kg/m2 (SD 3.02, min–max 19.8–29.5 kg), respectively. All 10 patients were on a CFTR modulator therapy prior to switching to ETI (five of these patients were on ivacaftor, and five patients were on lumacaftor/ivacaftor). At the time of self-reported mental health issues, follow-up clinical parameters in this group of patients on full standard dose of treatment (as seen in Table 1) showed significant improvement in lung function (ppFEV1 was 14.3 points higher compared to baseline, p = 0.0134), and significant reduction in sweat chloride (mean of difference in sweat chloride was −39.3 mmol/L, p < 0.005), consistent with the findings of the clinical trials (Heijerman et al., 2019; Middleton et al., 2019; Barry et al., 2021). As a result, all patients wanted to stay on ETI treatment given the significant improvements they experienced with respiratory clinical parameters.
TABLE 1.
Comparison of clinical efficacy parameters pretreatment, on full dose, and reduced dose of elexacaftor/tezacaftor/ivacaftor therapy.
| Genetic mutation | Pre- ETI treatment sweat chloride | Pre-ETI treatment FEV-1 | Sweat chloride on full dose ETI | FEV-1 on full dose ETI | Sweat chloride at 4–6 weeks (on reduced dose ETI) | FEV-1 at 4–6 weeks (on reduced dose ETI) | Sweat chloride at 10–12 weeks (on reduced dose ETI) | FEV-1 at 10–12 weeks (on reduced dose ETI) | Outcome of self-reported mental AEs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Delta F508/Delta F508 | 95 mmol/L | 3.8 L/79% predicted | 39 mmol/L | 4.27 L/107% predicted | 46 mmol/L | 4.20 L/106% predicted | 46 mmol/L a | 4.23 L/106% predicted a | Complete resolution |
| Patient 2 | Delta F508/G551D | — | — | 19 mmol/L | 2.67 L/65% predicted | 21 mmol/L | 2.72 L/70% predicted | 17 mmol/L a | 2.7 L/69% predicted a | Complete resolution |
| Patient 3 b | Delta F508/Delta F508 | 62 mmol/L | 3.92 L/92% predicted | 30 mmol/L | 4.04 L/96% predicted | 28 mmol/L | 4.06 L/96% predicted | 20 mmol//L c | 4.14 L/98% predicted c | Complete resolution |
| Patient 4 b | Delta F508/Delta F508 | 85 mmol/L | 3.87 L/85% predicted | — | — | — | — | 45 mmol/L d | 4.49 L/98% predicted d | Complete resolution |
| Patient 5 | Delta F508/G551D | 51 mmol/L | 2.73 L/69% predicted | 37 mmol/L | 3.78 L/95% predicted | 29 mmol/L | 3.98 L/101 % predicted | 32 mmol/L a | 4.26 L/108% predicted a | Complete resolution |
| Patient 6 | Delta F508/Delta F508 | 74 mmol/L | 3.36 L/84% predicted | 56 mmol/L | 3.32 L/83% predicted | 41 mmol/L | 3.40 L/85% predicted | 51 mmol/L a | 3.21 L/81% predicted a | Partial resolution |
| Patient 7 | Delta F508/Delta F508 | 105 mmol/L | 2.06 L/53% predicted | 39 mmol/L | 2.47 L/64% predicted | 45 mmol/L | 2.34 L/61% predicted | 56 mmol/L c | 2.35 L/61% predicted c | Complete resolution |
| Patient 8 | Delta F508/Delta F508 | 89 mmol/L | 1.3 L/48% predicted | 19 mmol/L | 1.83 L/68% predicted | — | — | 15 mmol/L c | 1.71 L/63% predicted c | Partial resolution |
| Patient 9 | Delta F508/G551D | 47 mmol/L | 1.62 L/56% predicted | 28 mmol/L | 1.79 L/68% predicted | 19 mmol/L a | 1.70 L/65% predicted | 24 mmol/L a | 1.77 L/67% predicted a | Partial resolution |
ETI, Elexacaftor/Tezacaftor/Ivacaftor; FEVI, Forced Expiratory Volume in 1 s; AEs, Adverse Events.
One tablet elexacaftor/tezacaftor/ivacaftor (100/50/75 mg) morning, and one tablet Ivacaftor 150 mg evening.
Initial complete discontinuation of ETI prior to dose reduction strategy.
Two tablets elexacaftor/tezacaftor/ivacaftor (200/100/150 mg) morning, (patients self-deviated from original plan).
One Tablet elexacaftor/tezacaftor/ivacaftor (100/50/75 mg) morning (Patient opted to remain on this dose given that he developed significant mental/psychological AEs within 2 weeks of standard-dose treatment initiation that necessitated hospital admission).
Patient 3: ETI therapy discontinued for 4 weeks, sweat chloride and ppFEV-1 were 99 mmol/L and 93%, respectively at the end of the washout period.
Patient 4: Patient opted to switch CFTR modulator therapy back to lumacaftor/ivacaftor, sweat chloride and ppFEV-1 while on lumacaftor/ivacaftor were 85 mmol/L and 91%, respectively. ETI therapy recommenced on reduced dose 4 months after initial experience.
This subgroup of patients (n = 10) was fully assessed by our multidisciplinary team. Patients who consented for further psychological assessment were referred to the appropriate service for psychological support and/or pharmacological therapy if indicated. Of those with reported mental health AEs, four patients were referred for psychological support, and required anxiolytic medications transiently that were discontinued within 2 weeks. None of the patients required long term psychotropics given resolution of AEs shortly after ETI discontinuation or dose reduction.
2.2 Dose reduction approach
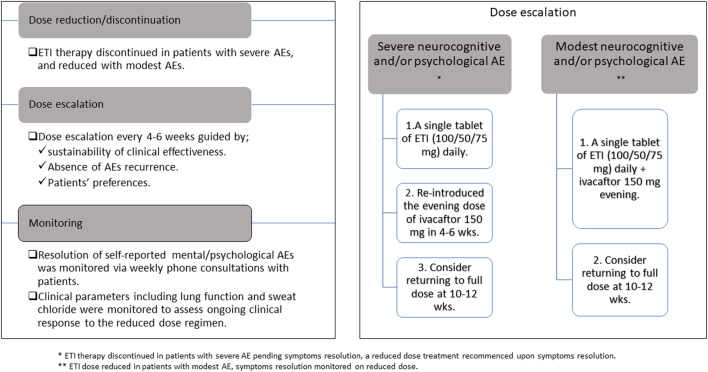
We adopted a dose reduction approach (Figure 1) in an attempt to minimize self-reported AEs but sustain adequate CFTR modulation. Our approach to dose reduction was developed in consultation with the CF multidisciplinary team and was as follows:
• In patients with severe self-reported neurocognitive and/or psychological AEs, we discontinued therapy pending AEs resolution. We subsequently re-introduced therapy at a lower dose, starting with a single tablet of ETI (100/50/75 mg) daily. We planned to reintroduce the evening dose of ivacaftor 150 mg at 4–6 weeks and considered returning to full dose therapy at 10–12 weeks. Dose escalation was guided by ongoing monitoring of clinical efficacy parameters, absence of psychological/neurocognitive AEs and patients’ preferences.
• In patients with modest self-reported AEs, we reduced CFTR modulator therapy dose to a single tablet of ETI (100/50/75 mg) daily and continued the evening dose of ivacaftor 150 mg. We considered returning to full dose therapy at 10–12 weeks.
FIGURE 1.
Outline of elexacaftor/tezacaftor/ivacaftor dose reduction approach.
To assess ongoing clinical response to the reduced dose regimen, clinical efficacy parameters including lung function (clinical marker of sustained response), and sweat chloride (indirect measure of CFTR function restoration) (Mall et al., 2020) were recorded every 4–6 weeks for the first 12 weeks on reduced dose therapy. Members of the CF multidisciplinary team (CF clinical nurse specialist) contacted these patients weekly via phone to monitor any changes in self-reported mental/psychological AEs. The assessment of AEs resolution was subjective based on patients’ perception of symptoms.
2.3 Response to reduced dose regimen
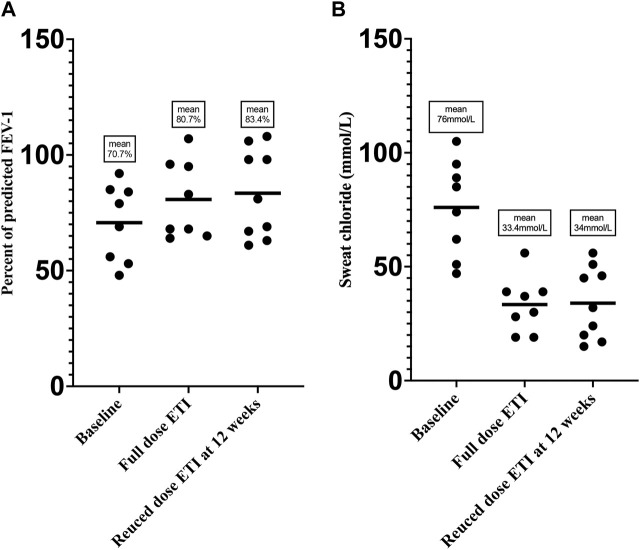
A total of nine patients commenced the dose reduction protocol and the remaining one patient opted to discontinue ETI and switched back to ivacaftor. Table 1 and Figure 2 demonstrates individual patient responses and dosing. Follow-up data showed resolution of self-reported mental/psychological AEs within 2 weeks in most patients, while their clinical efficacy parameters at 4–6 and 10–12 weeks on reduced dose were comparable to those on original full dose (mean ppFEV1 was 80.7% on standard dose ETI, compared to 83.4% at 12 weeks on reduced dose; mean sweat chloride was 33.4 and 34 mmol/L on standard and at 12 weeks on reduced dose, respectively).
FIGURE 2.
Change in (A) FEV1 and (B) sweat chloride at baseline, on full standard dose, and reduced dose of ETI therapy.
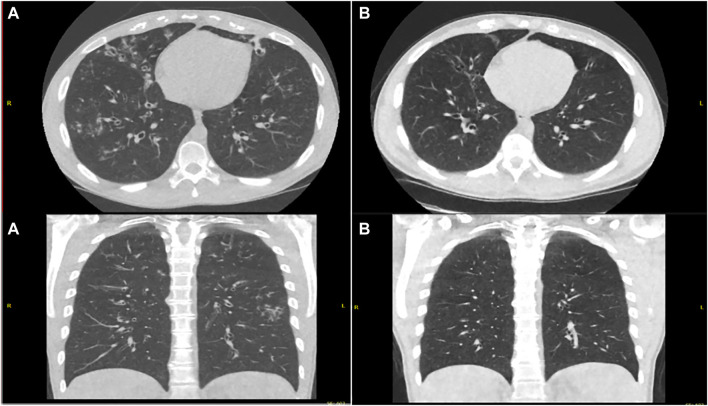
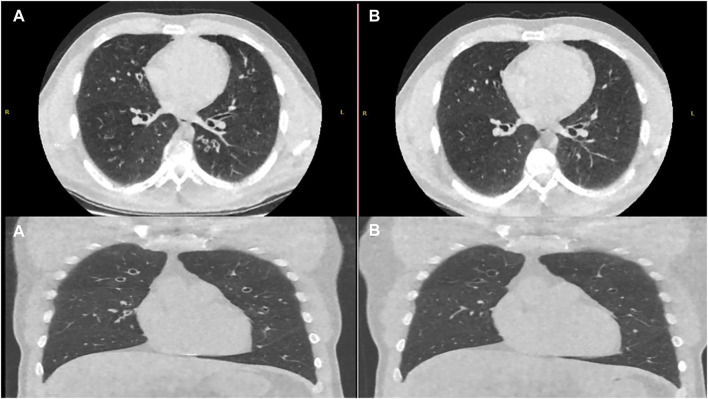
Whilst all nine patients adopted a sustained dosed reduction strategy, we acknowledge that two patients self-deviated from our proposed approach as highlighted in Table 1. At 12 weeks of the reduced dose regimen, six patients elected to remain on a reduced dose regimen and three patients switched back to full dose treatment. Repeat imaging in patients who completed 24 weeks on reduced dose regimen at the time of preparation of this manuscript (n = 2 of six patients) showed a significant improvement compared to imaging before initiation of modulator therapy (Figures 3, 4).
FIGURE 3.
Ultra-Low Dose CT Thorax pretreatment (A) and post modified dose regimen (B) showing reduced burden of bronchiectasis, reduced caliber of bronchiectatic airways, decreased bronchial wall thickening and resolution of tree in bud opacification changes.
FIGURE 4.
Axial and Coronal Ultra-Low Dose CT Thorax pretreatment (A) and post modified dose regimen (B) showing reduction in the degree of bronchial wall thickening and mucoid impaction.
3 Discussion
Over a 12 weeks period, dose reduction in our cohort of patients who developed self-reported mental health AEs resulted in the resolution of AEs without significant change to the clinical response achieved while on full dose of therapy. In addition, two of these patients had radiological improvement at 24 weeks on this regimen. That said, the long-term outcomes of reduced dose of ETI therapy remain unclear. Continued close real-world monitoring of this group is critical and ongoing.
We believe the mental health AEs in our cohort may be related to the add on effect of the CFTR correctors elexacaftor and/or tezacaftor, as all patients in this subgroup were previously on either ivacaftor or lumacaftor/ivacaftor prior to ETI therapy. One of the hypothesized mechanisms behind the mental health AEs of ETI therapy is drug-drug interaction between ETI and psychotropic medications, but none of the patients in this group were on any psychotropic medications, this would suggest that the mental health AEs in our cohort are likely directly related to ETI therapy. Furthermore, the mental health AEs resolved shortly after dose reduction or discontinuation of ETI therapy. A recently published case series outlining the real-world experience of a UK CF centre not only demonstrated a similar experience with the self-reporting of mental health AEs, but also a similar incidence of 7.1% (Spoletini et al., 2022). This further supports our belief that these events may be related to ETI therapy. Moreover, the UK case series also exemplifies that dose adjustment of ETI can improve mental health AEs while sustaining clinical effectiveness, which further validates our finding and approach (Spoletini et al., 2022). Currently, there is no standardised dose reduction strategy, we attempted to achieve this but even in our case series patients deviated in their approach to dose reduction. Moving forward, as we understand more about this possible AEs a standardised strategy would be helpful to the clinical community.
Based on recent real-world analysis of serum levels of ETI in 78 adult CF patients during routine outpatient visits, 41.1% (n = 37) of patients had elevated serum levels of elexacaftor compared to known Pharmacokinetic (PK) values of elexacaftor (Naehrig et al., 2022). This raises the question: are we reducing the dose of ETI in our cohort of patients with self-reported mental health AEs, or are we just simply optimizing the treatment dose? The access to routine drug levels for CFTR modulators remains an issue for clinical services and access to these levels could answer this question. In addition, it could allow for further optimization of dose reduction strategies for patients with self-reported AEs.
The limitations of this work is that it reflects a single centre experience, with a modest number of cases reported. The roll out of ETI therapy was during the COVID-19 pandemic, which enforced social restrictions and isolation on vulnerable populations, such as CF patients. This in addition to concerns regarding the effect of COVID-19 on CF patients health, may have contributed to self-reported anxiety among our patients. That said, one could also hypothesise that the close monitoring of these patients after ETI dose reduction at a time where a social restriction measure was imposed on them, may have had a placebo effect in reassuring some of these patients and contributed to the improvements seen in their mental health status post ETI dose modification. However, the temporal relationship between drug initiation and AEs makes us believe that this happened independently of the pandemic. Also, the rapid and significant change in the wellbeing of CF patients post CFTR modulator therapy, may require patients to make changes to lifestyle and future planning which could potentially contribute to the self-reported anxiety.
Our real-world data and published work to date highlight that some patients do not tolerate standard full dose of CFTR modulator therapy. There is a need in small groups of patients who develop AEs, to individualize dosage so as to minimize AEs whilst continuing CFTR modulator therapy. Access to routine drug levels for ETI will compliment clinical/sweat chloride monitoring and guide dose adjustment. Routine drug levels for ETI are not made routinely available to the clinical CF community, which may potentially prohibit CF clinicians from prescribing lower dose regimens to patients who develop neuropsychiatric and/or neurocognitive AEs, for instance. Increased awareness and reporting of real-world adverse events from CFTR modulators is critical.
Acknowledgments
The authors would like to acknowledge the Cork Adult CF Multidisciplinary Team for their contribution to this case series.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors on reasonable request, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Clinical Research Ethics Committee of the Cork Teaching Hospitals (CREC). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
The authors (HI, HD, DVM, KD, MMc, JD, CF, CH, ST, and TV) contributed to data collection and drafting the report. DMM and MM contributed to clinical and radiological monitoring of cases and aided report preparation. BP contributed to monitoring of cases and dose reduction strategy, data analysis and interpretation. All authors contributed to drafting the work and final approval.
Conflict of interest
BP has received both speakers’ fees and consulting fees from Vertex Pharmaceutical, Chiesi Pharmaceutical and Viatris Pharmaceutical, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Barry P. J., Mall M. A., Alvarez A., Colombo C., de Winter-de Groot K. M., Fajac I., et al. (2021). Triple therapy for cystic fibrosis phe508del-gating and -residual function genotypes. N. Engl. J. Med. 385 (9), 815–825. 10.1056/NEJMoa2100665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais R. V. E., Su V. C. H., Quon B. S. (2020). Real-world safety of CFTR modulators in the treatment of cystic fibrosis: A systematic review. J. Clin. Med. 10 (1), 23. 10.3390/jcm10010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijerman H. G. M., McKone E. F., Downey D. G., Van Braeckel E., Rowe S. M., Tullis E., et al. (2019). Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 394 (10212), 1940–1948. 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S., Young D. C., Safirstein J., Bourque B., Antell M. H., Diloreto S., et al. (2022). Mental status changes during elexacaftor/tezacaftor/ivacaftor therapy. J. Cyst. Fibros. 21 (2), 339–343. 10.1016/j.jcf.2021.10.002 [DOI] [PubMed] [Google Scholar]
- Hilliard M. E., Eakin M. N., Borrelli B., Green A., Riekert K. A. (2015). Medication beliefs mediate between depressive symptoms and medication adherence in cystic fibrosis. Health Psychol. 34 (5), 496–504. 10.1037/hea0000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. L., Noah T. L., Henry M. M. (2016). Therapeutic challenges posed by critical drug-drug interactions in cystic fibrosis. Pediatr. Pulmonol. 51 (S44), S61–S70. 10.1002/ppul.23505 [DOI] [PubMed] [Google Scholar]
- Keating D., Marigowda G., Burr L., Daines C., Mall M. A., McKone E. F., et al. (2018). VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 379 (17), 1612–1620. 10.1056/NEJMoa1807120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchford G., Duff A. J. (2013). Screening for depression in a single CF centre. J. Cyst. Fibros. 12 (6), 794–796. 10.1016/j.jcf.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Mall M. A., Mayer-Hamblett N., Rowe S. M. (2020). Cystic fibrosis: Emergence of highly effective targeted therapeutics and potential clinical implications. Am. J. Respir. Crit. Care Med. 201 (10), 1193–1208. 10.1164/rccm.201910-1943SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcorelles P., Friocourt G., Uguen A., Lede F., Ferec C., Laquerriere A. (2014). Cystic fibrosis transmembrane conductance regulator protein (CFTR) expression in the developing human brain: Comparative immunohistochemical study between patients with normal and mutated CFTR. J. Histochem Cytochem 62 (11), 791–801. 10.1369/0022155414546190 [DOI] [PubMed] [Google Scholar]
- McKinzie C. J., Goralski J. L., Noah T. L., Retsch-Bogart G. Z., Prieur M. B. (2017). Worsening anxiety and depression after initiation of lumacaftor/ivacaftor combination therapy in adolescent females with cystic fibrosis. J. Cyst. Fibros. 16 (4), 525–527. 10.1016/j.jcf.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Middleton P. G., Mall M. A., Drevinek P., Lands L. C., McKone E. F., Polineni D., et al. (2019). Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 381 (19), 1809–1819. 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naehrig S., Breuling M., Behr J., Habler K., Paal M. (2022). WS17.01 Therapeutic drug monitoring of elexacaftor, tezacaftor and ivacaftor in adult patients with cystic fibrosis. J. Cyst. Fibros. 21, S32. 10.1016/s1569-1993(22)00249-1 [DOI] [Google Scholar]
- Quittner A. L., Abbott J., Georgiopoulos A. M., Goldbeck L., Smith B., Hempstead S. E., et al. (2016). International committee on mental health in cystic fibrosis: Cystic fibrosis foundation and European cystic fibrosis society consensus statements for screening and treating depression and anxiety. Thorax 71 (1), 26–34. 10.1136/thoraxjnl-2015-207488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner A. L., Goldbeck L., Abbott J., Duff A., Lambrecht P., Sole A., et al. (2014). Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: Results of the international depression epidemiological study across nine countries. Thorax 69 (12), 1090–1097. 10.1136/thoraxjnl-2014-205983 [DOI] [PubMed] [Google Scholar]
- Schneider E. K., McQuade R. M., Carbone V. C., Reyes-Ortega F., Wilson J. W., Button B., et al. (2018). The potentially beneficial central nervous system activity profile of ivacaftor and its metabolites. ERJ Open Res. 4 (1), 00127–02017. 10.1183/23120541.00127-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell C., Fernandes S., Bujoreanu I. S., Garcia G. (2014). Depression, illness severity, and healthcare utilization in cystic fibrosis. Pediatr. Pulmonol. 49 (12), 1177–1181. 10.1002/ppul.22990 [DOI] [PubMed] [Google Scholar]
- Spoletini G., Gillgrass L., Pollard K., Shaw N., Williams E., Etherington C., et al. (2022). Dose adjustments of Elexacaftor/Tezacaftor/Ivacaftor in response to mental health side effects in adults with cystic fibrosis. J. Cyst. Fibros. 21 (6), 1061–1065. 10.1016/j.jcf.2022.05.001 [DOI] [PubMed] [Google Scholar]
- Sutharsan S., McKone E. F., Downey D. G., Duckers J., MacGregor G., Tullis E., et al. (2022). Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: A 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir. Med. 10 (3), 267–277. 10.1016/S2213-2600(21)00454-9 [DOI] [PubMed] [Google Scholar]
- Talwalkar J. S., Koff J. L., Lee H. B., Britto C. J., Mulenos A. M., Georgiopoulos A. M. (2017). Cystic fibrosis transmembrane regulator modulators: Implications for the management of depression and anxiety in cystic fibrosis. Psychosomatics 58 (4), 343–354. 10.1016/j.psym.2017.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors on reasonable request, without undue reservation.