Abstract
Immunoglobulin G4-related disease (IgG4-RD) is a chronic multi-organic immune fibrosing disease. It affects preferentially men around middle age and almost any organs can be involved; however, lymph nodes, submandibular and lacrimal glands, pancreas, and retroperitoneum are the most affected. The mainstay treatment is corticosteroids, sometimes adjuncts with DMARDs or rituximab as steroid sparing agents. Th2 inflammation is implicated in the pathophysiology of the disease. Several reports indicate that allergy and/or atopy often affect patients with IgG4-RD. The frequency varies greatly between studies with allergies/allergic diseases reported in 18–76% while atopy is reported in 14–46%. In studies including both, they affect 42 and 62% of patients. Rhinitis and asthma are the most frequent allergic diseases. IgE and blood eosinophiles are often elevated and few studies report that basophils and mast cells could participate in the disease pathogenesis; however, the implication of allergy and atopy remain unclear. No common allergen has been identified and IgG4 production seems to be polyclonal. Although a direct causal effect is unlikely, they could potentially shape the clinical phenotype. Allergies/allergic diseases and/or atopy are reported to be more frequent in IgG4-RD patients presenting head, neck, and thoracic involvement, with higher IgE and eosinophils and less frequent in retroperitoneal fibrosis; however, studies regarding allergy and atopy in IgG4-RD are highly heterogenous. The aim of this article is to review what is currently known about the allergy and atopy in the context of Ig4-RD.
Keywords: IgG4-related disease, Allergy, IgE, Eosinophils, Th2 cells
Immunoglobulin G4-related disease (IgG4-RD) is a recently described multi-organic immune fibrosing disease.1 It is characterized histopathologicaly by dense IgG4+ lymphoplasmocytes infiltrates, storiform fibrosi,s and obliterans phlebitis.2 Affecting preferentially middle age/older men, it most frequently involved lymph nodes, submandibular and lacrimal glands, pancreas, and retroperitoneum. However, it can affect almost any organs, from pituitary glands to prostate. Therapeutic options are for now limited to corticosteroids, DMARDs, and rituximab.3 There is still no targeted treatment available. Advances in the last decade showed that Th2 inflammation is implicated in the pathophysiology of the disease. Th2 cytokines are elevated, as well as IgG4, IgE and eosinophils4 Allergy and atopy are also often present in patient with IgG4-RD. However, a question persists around the implication of allergy and atopy in the pathogenesis of the disease. The aim of this article is to review what is currently known about the allergy and atopy in the context of Ig4-RD.
The importance of IgG4 in allergy
IgG4 is a peculiar antibody, its exact role is not completely understood. It is the only IgG subclass to bind to both activating Fcγ receptors and inhibitory FcγRIIb.5 It does not bind FcγRIIIb and does not activate the complement. IgG4 can also be modified by Fab-arm exchange (FAE). Under certain conditions, the 2 heavy chains can dissociate and re-associate with a different half-molecule, hence forming bi-specific monovalent antibodies.6 This form represents around 20–30% of IgG4 in humans.7 IgG4 are produced by B cells after isotype class-switching under the influence of Th2 cytokines IL-4 and IL-13, which also induce class-switching to IgE. The conjunction with IL-10 and IL-21 orients the process. Under certain circumstances, IL-10 enhance the production of IgG4 and reduced IgE.8 In vitro, adjunct of IL-21 reduces IgE production while enhancing IgG4.9
In the context of allergy, IgG4 are associated with the development of tolerance. The occurrence of allergy versus immune tolerance depends on the balance between IgG4 and IgE production. In the modified Th2 hypothesis, a high dose chronic allergen-driven Th2 response enhances IgG4 production and dominates over IgE production, hence protecting from immediate hypersensitivity.10,11 In the context of allergen immunotherapy (AIT), there is an allergen driven IL-10 production by peripheral blood mononuclear cells.12 Allergen specific IgG4 production occurs 6–8 weeks after initiation of therapy13,14 IgG4 blocks the liaison of IgE-allergen complexes to FcεRII of antigen-presenting cells, thus preventing presentation to T cells.15 It also prevents the signaling of IgE through FcεRI via direct competition by binding to the allergen, and possibly via stimulation of inhibitory FcγRIIb, thus inhibiting immediate hypersensitivity.16
Definition of allergy and atopy in studies
Before reviewing the literature about allergy in IgG4-RD, it is important to keep in mind that different definitions are used to define either allergic or atopic groups in studies. Allergy and atopy are not synonymous. Allergy is “a hypersensitivity reaction initiated by specific immunologic mechanisms”, either IgE- or non-IgE mediated. Atopy represents “a personal and/or familial tendency, usually in childhood or adolescence, to become sensitized and produce IgE antibodies in response to ordinary exposures to allergens, usually proteins. As a consequence, these persons can develop typical symptoms of asthma, rhinoconjunctivitis, or eczema”.17 Atopy should therefore be used to define IgE-antibody high-responders, patients who develop IgE to commonly encountered allergen to which normal population do not become sensitized. Patients sensitized to Hymenoptera venom or drugs, for example, should not be considered atopic.
Definitions to identify allergy and/or atopy differs in studies. The allergy definition of the European Academy of Allergy & Clinical Immunology (EAACI), atopy definition of EAACI, and unofficial definitions are used. In some studies, definitions are not mentioned (Table 1). Except in one study, allergic diseases were not clearly confirmed by an allergist.18
Table 1.
Definitions of allergy/atopy used in studies
| Atopy: EAACI19, 20, 21, 22, 23 |
| Atopy: Evidence of an IgE antibody response in addition to clinical symptom4 |
| Allergy and/or Atopy: EAACI24,25 |
| Allergy: EAACI26, 27, 28, 29, 30, 31 |
| Allergy: To have allergic rhinitis, allergic asthma, or allergic skin disease, such as urticarial history, etc.32 |
| Allergy: Allergic diseases, such as acute allergic rhinitis, atopic dermatitis, and bronchial asthma33 |
| Allergy: Allergic disorders: hay fever and allergic rhinitis in addition to asthma34 |
| Allergy: bronchial asthma, acute allergic rhinitis, hay fever and atopic dermatitis35 |
| Allergy: symptomatic allergic conditions diagnosed by a physician at diagnosis36 |
| Allergic rhinitis was diagnosed on the basis of medical history, symptoms, nasal cytology, and specific IgE to aeroallergens.37 |
| Allergy: allergic disease, including inhalation allergy, drug allergy, food allergy, contact allergy, and “;irritable physique”18 |
EAACI: European Academy of Allergy and Clinical Immunology.
Prevalence and characterization of allergy and atopy in patients with IgG4-related disease
The prevalence of allergy/allergic diseases and atopy varies greatly between studies. Allergies/allergic diseases are described in 18–76% of IgG4-RD patients,18,25, 26, 27, 28, 29, 30, 31, 32,36,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 while atopy is reported in 14–46%.19, 20, 21, 22, 23,50,51 Two studies included both allergic and atopic patients in the same group with a prevalence of 42% and 62%, respectively.4,24 While this review is neither a systematic review nor a meta-analysis, we have attempted to compare the different data in forest plots for random effects meta-analysis to have a general idea of the situation (Fig. 1, Fig. 2). The mean prevalence of allergies/allergic disease and atopy are respectively 49% (95% CI [42–55%]) and 29% (95% CI [20–40%]). In cohorts of autoimmune pancreatitis (AIP), the frequency of allergy/allergic disease reported is from 15 to 44%.33, 34, 35,52 One study reported that allergy was more frequent in patients from 40 to 59 years old (55-54%) compared to patients younger (49%) or older (39–49%).27
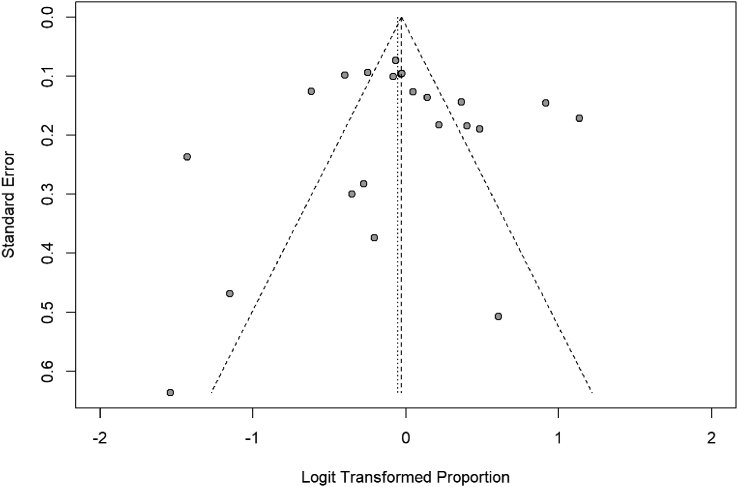
Fig. 1.
Proportion of allergy/allergic diseases in patients with IgG4-RD. Forest plot. Random effects model: proportion 0,49 [0,42; 0,55]. Heterogeneity: I2 = 90%, p < 0.01
Fig. 2.
Funnel plot 1. Funnel plot of studies included to calculate the proportion of allergy/allergic diseases in patients with IgG4-RD
Rhinitis is the most frequent allergic disease followed by asthma (Table 2). Rhinitis is reported in 2–31%18, 19, 20, 21,25,28,36,44,50,53,54 of patients with IgG4-RD; most studies report rates above 20%. Asthma is described in 4–27%.18, 19, 20, 21,28,36,44,46,50,53,54 Conjunctivitis affected 7–9% of subjects.19,21,36,47 In AIP, rhinitis and asthma affect 7–22%33,35,52 and 7–12% of patients, respectively (Table 2).33, 34, 35,52 Subjects with dacryoadenitis and sialadenitis (DS) have the highest frequency of rhinitis and conjunctivitis (41–52% and 20%, respectively) (Table 2). Asthma is reported in 9–14% of subjects.55, 56, 57 Urticaria, eczema/atopic dermatitis, and contact dermatitis were rarely reported in patients with IgG4-RD. Skin allergies otherwise not specified were reported in 13–42% of patients.25,54 Food allergy was reported in 1–20%18,19,21,25,35,44,50 and drug allergy in 2–20%.18,28,33,35,44,50,53 Anaphylaxis is reported in 2 studies in 3% and 9% of patients.25,50 Venom allergy was not reported.
Table 2.
Allergic manifestations in IgG4-RD.
| Rhinitis | Conjunctivitis | Asthma | Food allergy | Drug allergy | Anaphylaxis | Others | |
|---|---|---|---|---|---|---|---|
| Saeki 201836 22/51 (43%) allergic conditions |
15 (29%) | 4 (8%) | 16 (25%) | – | – | – | – |
| Sanders 202025 165/231 (71%) allergic conditions |
Aero-allergens symptoms: 135 (58%) | 47 (20%) | – | 20 (9%) | Skin allergya: 927 (42%) |
||
| Zhou 202018 201/459 (44%) allergic diseases |
99 (22%) | – | 20 (4%) | 3 (1%) | 30 (7%) | – | Contact allergy: 7 (2%) Mixed allergies: 11 (6%) “Irritable the physique”: 8 (2%) |
| Lin 201544 73/118 (62%) allergy |
33 (28%) | – | 33 (28%) | 6 (5%) | 24 (20%) | – | Skin sensitivity or urticaria: 10 (8%) |
| Zen 201028 22/114 (19%) allergic disorders |
Sinusitis: 4 (4%) Rhinitis: (2%) |
– | 14 (12%) | – | 2 (2%) | – | – |
| Della Torre 201419 22/70 (31%) atopy |
16 (23%) | 5 (7%) | 8 (11%) | OAS: 1 (1%) | – | 0 (0%) | Urticaria: 3 (4%) |
| Della-Torre 202050 29/116 (25%) atopy |
14 (12%) | 10 (9%) | aeroallergen: 6 (5%) food and drugs: 9 (8%) |
3 (3%) | 18 (16%) | 3 (3%) | Contact allergy: 3 (2%) Urticaria/angioedema: 10 (9%) Dermatitis: 6 (5%) GI symptoms: 1 (1%) |
| Mattoo 201420 18/39 (46%) atopy |
12 (31%) | 3 (8%) | 5 (13%) | – | – | 0 (0%) | Urticaria: 3 (8%) GI symptoms: 1 (3%) |
| Mattoo 201621 33/74 (45%) atopy |
18 (24%) | 5 (7%) | 9 (12%) | OAS: 1 (1%) | – | 0 (0%) | Urticaria: 3 (4%) Eczema: 2 (6%) “Hay fever”: 2 (6%) |
| Inoue 201553 – |
29 (12%) | – | 25 (11%) | – | 16 (7%) | – | – |
| Matsui 201854 – |
20 (30%) | – | 18 (27%) | – | – | – | Skin allergya: 9 (13%) |
| AIP | |||||||
| Hirano 201052 7/42 (16%) allergic conditions |
3 (7%) | – | 3 (7%) | – | – | – | Urticaria: 1 (2%) |
| Kamisawa 200933 20/45 (44%) allergic conditions |
11 (24%) | – | 3 (7%) | – | 2 (4%) | – | Hypersensitivity pneumonitis: 1 (2%) |
| Sah 201034 12/78 (15%) |
– | – | 9 (12%) | – | – | – | Others: 3 (4%) |
| Kuruma 201435 24/67 (36%) |
15 (22%) | – | 8 (12%) | 1 (2%) | 4 (6%) | – | Atopic dermatitis: 2 (3%) House dust allergy: 1 (2%) Hamster allergy: 1 (2%) |
| DS | |||||||
| Yamamoto 201955 165/281 (59%) allergy |
140 (50%) | 56 (20%) | 25 (9%) | – | – | – | Urticaria: 5 (2%) |
| Masaki 200956 – |
26 (41%) | – | 9 (14%) | – | – | – | – |
| Li et al. 201557 – |
22 (52%) | – | 6 (14%) | – | – | – | – |
Otherwise non specified, GI, Gastro-intestinal; NR, Not reported; OAS, Oral allergen syndrome
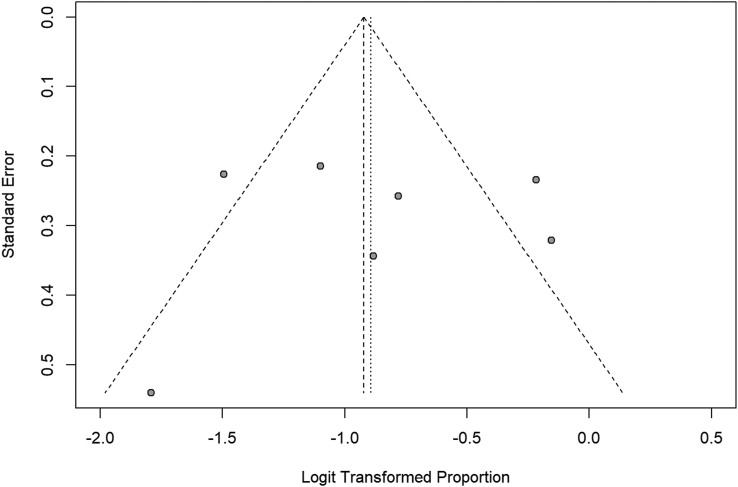
While we attempted to compare the different studies, several factors make the comparison difficult. Studies are highly heterogenous in both allergic and atopic groups (I2 = 90%, p < 0.01 and I2 = 76%, p < 0.01, respectively). Funnel plots of the allergic group seem asymmetrical, and some studies are outside of the funnel zone as showed in Fig. 3. The funnel plot of the atopic group seems more symmetrical (Fig. 4), but this conclusion could be influenced by the small number of studies collected. There could be publication bias; however, since we are in the presence of heterogeneity, funnel plots need to be interpreted carefully. Moreover, studies are prone to multiple limitations. Definitions used to identify allergic/atopic patients differ between studies, and specific allergic diseases researched/diagnostic criteria of each condition are not always known. Most diagnoses were either made by file reviewing or written questionnaire. There was no mention of direct evaluation by either an allergist, a respirologist, or a dermatologist, except in 1 study.18 It is not known if allergy diagnosis was supported by specific IgE (sIgE) or skin testing or asthma supported by pulmonary functional test (PFT) or methacholine challenge. There are also disparities regarding terminology. By example, some reported “Hay fever”, “hamster allergy”, and “house dust allergy” in addition to rhinitis, conjunctivitis, and asthma.20,35 Those patients should have been included in the later categories. Classifying patients according to their allergens instead of the symptoms that occur could be interesting. In cases of anaphylaxis and urticaria, the culprit agent should be reported. Urticaria should also be defined as acute or chronic. Also, “skin allergy” is an umbrella term representing different pathologies and should be avoided. Finally, “irritable the physique” should not be used to define allergy.
Fig. 3.
Proportion of atopy in patient with IgG4-RD. Forest plot. Random effects model: proportion 0.29 [0.20; 0.40]. Heterogeneity: I2 = 76%, p < 0.01
Fig. 4.
Funnel plot 2. Funnel plot of studies included to calculate the proportion of atopy in patient with IgG4-RD
It remains unclear if allergy/atopy is more frequent in IgG4-RD compared to the general population. Many studies do not include a control group,18, 19, 20, 21, 22,24,25,28, 29, 30,32,34, 35, 36,38,40, 41, 42,46,51,52,54 and few studies compared results to "historical" cohorts. Della-Torre et al and Campochiaro et al showed allergy/atopy prevalence that was equivalent to the general population.19,23 On the contrary, Saeki and al reported that allergic conditions were more frequent in IgG4-RD;36 however, populations in those studies were not directly compared. Also, choice of comparative population can be biased. Few studies included a control group and results were also discording. When comparing to healthy control (HC), Culver et al (62.5% vs 17.1%; p < 0.0001) and Sah et al (15% vs 4%%; p = 0.006) demonstrated a higher frequency of atopy and allergic manifestations.4,34 However, Culver et al reported that it was identical in another, but smaller study involving 24 patients.24 Compared to Sjögren syndrome, IgG4-RD subjects had higher prevalence of allergic rhinitis (40.6% vs 17.2%; p = 0.001).57 The choice of comparison population is important. For example, Sanders et al concluded that prevalence of allergy in IgG4-RD was equivalent to the general population as they compared to controls.25 However, controls were patients without inflammatory disease from rheumatology clinic (osteoarthritis, fibromyalgia, osteoporosis) and allergy frequency was surprisingly high (79% IgG4-RD vs 71% controls). Indeed, according to World Allergy Organization (WAO), 10%–30% of adult population in the United States have 1 or more allergic disease.58 A few years earlier, Sanders et al had concluded that allergy in IgG4-RD was more frequent (76%) than the general population when they compared to a survey in which 30% of Americans were identified as being allergic.47,59
IgG4-RD organ involvement and allergy
Studies suggest that allergy and/or atopy in IgG4-RD is more frequent in patient with dacryoadenitis and/or sialadenitis (DS), ophthalmic disease, chronic rhinosinusitis (CRS), and intrathoracic involvement. Four studies demonstrated that allergies are more prevalent in IgG4-related dacryoadenitis and/or sialadenitis (IgG4-RDS).25,30,31,43 Liu et al and Chen et al respectively reported allergic diseases in 51% of sialadenitis vs 25% of non-sialadenitis (p < 0.001) and 64.6% of DS vs 43.9% in non-DS (p < 0.001).31,43 Patients with DS with or without other organ involvement had higher frequency of allergies compared to patients without DS (69.6% vs 61.8% vs 34.1%; p < 0.003). They are also more prone to rhinosinusitis symptoms and nasal and paranasal involvement.30 Sanders et al, in a cohort of 165 patients, showed an association between sialadenitis and/or dacryoadenitis and allergies (aOR: 1.92 [95% CI: 1.06–3.48]).25 Patients with IgG4 related ophthalmic disease (IgG4-ROD) also have higher frequency of allergy (66%) compared to patient without (26%).26 Moteki et al, demonstrated that patient with IgG4 related CRS have an allergy prevalence of 32%. Of those, 71% had allergic rhinitis diagnosed by nasal smear eosinophil and local findings and 10% had asthma.60 Gao et al reported that allergies were more frequent in IgG4 related CRS than in patient without (56.5% vs 20% [p = 0.004]). Skin allergies (20 vs 0%), asthma (30 vs 13%) and drug allergies (10 vs 0%) were also more frequent.49 In a cohort of IgG4-RDS, Pia et al found 25% of allergic rhinitis in patients with related CRS vs 0% in the group without. Bronchial asthma was also more frequent in the CRS group (25% vs 0%).61 Patients with intra-thoracic lesions also have higher prevalence of allergies (63.2% vs 44.7% without [p = 0.008]).32 In a study comparing 4 group of patients (pancreato-hepato-biliary disease vs retroperitoneal fibrosis (RPF) and/or aortitis vs head and neck-limited disease vs and classic Mikulicz's syndrome with systemic involvement), Lanzillota et al found more prevalent allergic diseases in the head and neck group, but it was not statistically significant (p = 0.09).51
Allergy testing in IgG4-RD
Allergy testing were used only in 5 studies (Table 3). Culver et al tested 48 patients with IgG4-RD (40% of atopic) with specific IgE (sIgE). Fifty-two percent had at least 1 elevated sIgE.4 Matsui et al retrospectively described 48 patients with sIgE to aeroallergens, 79% had at least 1 positive result.54 Yamamoto et al tested sIgE on 257 IgG4-RDS.55 One hundred sixty-three (63.4%) patients had sIgE against at least 1 allergen. D. pteronyssinus was the most identified (91 patients). Kuruma et al performed sIgE testing on 15 patients with AIP 87% had at least 1 positive response while only 7 had reported clinical allergic diseases.35 Della-torre et al are the only ones to have performed sIgE and skin prick test. On 10 atopic patients, they demonstrated sensitization to dust mite, mold, grass, ragweed, cat dander, and shellfish.19 No common culprit allergen could be identified in those studies.4,19,35,54,55 However, interpretation of those results is difficult. It is unclear whether patients were evaluated by allergists in all studies. Methods and cut-off values were not mentioned in many studies4,19,55 and levels of sIgE were never reported. It is also important to note that sIgE and skin prick test alone are insufficient evaluation of allergy and atopy. Those results were not reported taking into consideration allergic symptomatology. Elevated sIgE could be present because of a global elevation of serum IgE associated with IgG4-RD. As a matter of fact, Kuruma et al enquired about disproportion of patients with positive sIgE (87%) vs identified allergic patients (36%).35 Do they reflect only sensitization or true allergies? Indeed, the value of a positive sIgE in absence of symptoms is unknown. Interpretation of sIgE results requires correlation with the clinical picture.62
Table 3.
Allergy tests used in studies
| Methods | Allergen tested | |
|---|---|---|
| Culver et al.4 48 patients |
Specific IgE (ImmunoCap method) |
Grass, mold, tree and nut mixes 52% had at least one positive result |
| Matsui et al.54 48 patients |
Specific IgE (ImmunoCap method, Radioallergosorbent test) |
16 aeroallergens 79% had at least one positive result |
| Yamamoto et al.55 257 patients with IgG4-RDS |
Specific IgE (ImmunoCap method) |
Birch pollen, cedar pollen, moth scales, Dermatophagoides pteronyssinus, Poaceae pollens, weed pollens, food allergens, grain allergens, animal epithelia, and mold allergens 63.4% had at least one positive result |
| Kuruma et al.35 15 patients with AIP |
Specific IgE (Radioallergosorbent test (positive >0.34 UA/mL) Multiple allergosorbent test system (positive > class 1)) |
Not mentioned 87% had at least one positive result (Causative agents: cedar, Japanese cypress, ticks, shrimp, crab, orchard grass, house dust, moths, Ambrosia artemisiifolia, Candida, wheat, Aspergillus, guinea pig, mugwort, peanut, buckwheat, sweet vernal grass, timothy, Betula platyphylla and Penicillium) |
| Della-torre et al.19 10 atopic patients |
Specific IgE Skin tests |
Not mentioned Sensitization to dust mite, mold, grass, ragweed, cat dander and shellfish |
Differences between allergic/atopic and non-allergic/atopic IgG4-RD patients
It is still debated if patients with allergies and/or atopy form a particular group within IgG4-RD subjects and have distinct manifestations/laboratory values (Table 4). There is no difference between sex or age at presentation between patients with allergy/atopy and those without. Only 1 small study identified a female preponderance and younger age at presentation.36 Up to now, no studies showed differences in number of organs involved19,36,50,55 and rate of relapse.36 Regarding specific organs involved, results are conflicting. Two studies found no differences and 3 showed discordant results. Saeki et al showed that upper body (head, neck, and thoracic area) and lung were more involved, 80% vs 56% and 50% vs 31% respectively.36 In a Mattoo et al study, salivary glands (61 vs 38%), lacrimal glands (22 vs 10%), and orbits (22 vs 10%) were more involved.20 In a Della-Torre et al study, RPF was less frequent (5 vs 29%).19 Those studies were small and p values were not always available. However, it is concordant with previous studies showing that allergy/atopy is more frequent in patient with IgG4-RDS.25,30,31,43 Sanders et al demonstrated that patients with high IgG4 had more allergic manifestations,25 but no studies could identify a difference in serum IgG4 between allergic and non-allergic. Results about serum IgE are also conflicting. Four studies reported higher serum IgE and/or higher number of patients with elevated IgE,4,19,21,24,33 but 5 others did not. 22,36,43,50,52 Regarding serum eosinophils, 2 studies showed higher eosinophils or more eosinophilia in allergic/atopic patients.19,21 However, 4 others did not.22,23,36,50 Supplementary data from Mattoo et al suggest that atopic patients with IgG4-RD have higher serum IgG4 (26/33 [79%] vs 12/41 [29%]) and IgE (20/33 [45%] vs 6/41 [17%]), and more eosinophilia (15/33 [61%] vs 22/74 [15%]) than IgG4-RD patients without atopy21 P values were not mentioned in the text, but were statistically significant below 0.05 when calculated using Chi2 test (p = 0,0023; p = 0,0004 and p = 0,0079 respectively). Yamamoto et al demonstrated that sensitized patients were slightly younger (60 vs 65 years) and had higher serum IgE (612.66 ± 1604.96 vs 201.18 ± 285.91 IU/mL) than non-sensitized patients. They found no difference in regard of sex, organ involvement, IgG4, and eosinophils.55
Table 4.
Differences and similarities between allergic/atopic and non-allergic/atopic patients in IgG4-RD.
| Different parameter | Similar parameter | |
|---|---|---|
| Della-Torre et al., 2020 (Italy)50 29 atopic vs 87 non-atopic |
IgG4-RD RI: 8.3 (3.2) vs 9.6 (3.0); p = 0.01 | Sex and age Number of organs involved Specific organ involvement Serum IgG4 Blood eosinophils Serum IgE Plasmablasts |
| Saeki et al., 2018 (Japan)36 22 allergic conditions vs 29 without |
Sex: Male 8 (36.4) vs 21 (72.4); p = 0.010 Age: 61.0 ± 13.2 vs 71.6 ± 8.1; p = 0.002 Specific organ involvement: Lung: 11 (50%) vs 9 (31%); p = 0.032 Upper body affected organs: 80 ± 24 vs 56 ± 32; p = 0.004 |
Number of organs involved Serum IgG4 Blood eosinophils Serum IgE IgG4-RD RI Relapse |
| Chen et al., 2016 (China)43 118 allergic vs 82 non-allergic |
Serum IgE | |
| Culver et al., 2017 (UK)4 30 allergy/atopy vs 18 without |
Serum IgE: Higher IgE ∗ p < 0.05 | |
| Culver et al., 2015 (UK)24 10 allergy/atopy vs 14 without |
Serum IgE: Higher IgE ∗ p = 0.0255 | |
| Della-Torre et al., 2014 (USA)19 22 atopic vs 48 non-atopic |
Specific organ involvement: RPF: 5 vs 29%; p = NA Serum IgG4: None Blood eosinophils: 641 cells/μL versus 365 cells/μL; p = 0.02 Serum IgE: 454 mg/dL vs153 mg/dL; p = 0.01 |
Sex and age Number of organs involved |
| Mattoo et al., 2014 (USA)20 18 atopic vs 21 non-atopic |
Specific organ involvement: salivary glands 61.1 vs 38%; p = NA lacrimal glands 22.2 vs 10%; p = ? Orbits 22.2% vs 10%; p = NA |
Sex and age Serum IgG4 |
| Mattoo et al., 2016 (USA)21 74 atopic vs 27 non-atopic |
Serum IgG4: Elevated IgG4: 79% (26/33) vs 29% (12/41) Eosinophilia: 45% (15/33) vs 17% (7/41) Elevated IgE: 61% (20/33) vs 15% (6/41) |
Sex and age |
| Grados et al., 2017 (France)22 4 atopic vs 24 non-atopic |
Blood eosinophils Serum IgE |
|
| Campochiaro et al., 2016 (Italy)23 12 atopic vs 29 non-atopic |
Blood eosinophils Serum IgE |
|
| Hirano et al., 2010 (Japan)52 7 allergic vs 35 non-allergic AIP |
Serum IgE | |
| Kamisawa et al., 2009 (Japan)33 20 allergic vs 25 non-allergic AIP |
Specific organ involvement: Less obstructive jaundice and abdominal pain; p = 0.012 and p = 0.013 Eosinophilia (>600 cells/mm3): 25% vs 0%; p = 0.013 Eosinophil count: 292.50 vs 160 cells/mm3; p = 0.048 Elevated IgE (>580 IU/mL): 80% vs 0%; p < 0.0001 Serum IgE: 793.50 IU/mL vs 176 IU/mL; p = 0.00001 |
Sex and age Serum IgG4 |
| Yamamoto et al., 2019 (Japan)55 163 sensitized (82 allergic) vs 94 non-sensitized (32 allergic) DS |
Age: 60 vs 65 years old; p < 0.005 Serum IgE: 612.66 ± 1604.96 vs 201.18 ± 285.91 IU/mL; p < 0.05 |
Sex Number of organs involved Specific organ involvement Serum IgG4 Blood eosinophils |
AIP, Auto-immune pancreatitis; DS, Dacryoadenitis and sialadenitis; IgG4-RD RI, ImmunoglobulinG4-related disease responder index; NA, not available
IgE in IgG4-RD
Total serum IgE are elevated in most subjects with IgG4-RD.4,18,19,21,23,25,27,36,38,43,44,54,63,64 Thirty-five to 67% of patients have serum IgE higher or equal to 100 IU/mL.19,21,23,25,38 Matsui et al demonstrated that 89.4% had serum IgE above 170 U/mL54 and Saeki et al that 56% had more than 250 IU/mL.36 Chen et al reported that IgE level was increased in 83,6% of patient, but threshold was not indicated.43 In a large cohort of 425 patient, median IgE was 347 (IQR 126–752) KU/L.41 Similar values were identified in a smaller cohort in Japan (384.6 ± 565.4 IU/mL).37 In Occident, Della-torre et al reported a mean concentration of 523 IU/mL (range 129–1869)19 and Grados et al reported a median IgE level of 627 ± 1092 UI/l.22 IgG4-RD patients also have higher serum IgE than healthy subjects. Culver et al demonstrated IgE levels >125 kIU/L in 54% of IgG-RD vs 6% of HC; p < 0.001. Serum IgE was also increased in their high serum IgG4 group (median 323 kIU/L; range 3.31–2024) compared to normal IgG4 group (median, 55.7 kIU/L; range, 5.11–4457) (P = 0.003). It also had the ability to distinguish IgG4-RD from diseases control with elevated IgG4, area under the curve was 0.69 (p = 0.004; 95% CI, 0.57–0.81).4 IgE levels are also higher in IgG4-RD than Sjögren's Syndrome (SS) (307.4 vs 15.3 IU/mL; p = 0.005).56 In the AIP cohort, IgE are also elevated.35,52 Hirano et al had 86% of patients with elevated IgE with average value of 679 IU/mL (range, 67–3000).52 Levels of IgE are also high in patients with DS55,57 and tubulointerstitial nephritis.64 Lanzillotta et al showed that serum IgE were the highest in patient with pancreato-hepato-biliary disease and Mikulicz's syndrome with systemic involvement (median 283 and 219 IU/mL) and lowest in RPF and/or aortitis (median 69 IU/mL) (p = 0.02).51 Also, patients with ophthalmic involvement have elevated IgE more frequently than patient without. (87% vs 71%; p = 0.008).26
Patients with elevated total serum IgE have some distinguishing features. In a large cohort, Zhou et al compared 399 patients with elevated IgE (>60 KU/L) to 60 patients with normal level. Patients with higher IgE had more organ involved and higher IgG4-RD RI score. Submandibular glands and pancreas were the most frequently involved. Serum IgG4 and eosinophils were higher. Also, they had more frequent allergies (46,4% vs 26,7%; p = 0.004)18 However, this finding was not described by Sanders et al.25
Eosinophils in IgG4-RD
Eosinophils are also elevated in IgG4-RD; the percentage of patients with eosinophilia (defined as >500 cells/μL or >0,5 × 109/L) varies from 19 to 38%.4,19,21, 22, 23,25,27,36,41,43,44,65 When present, eosinophilia tends to be moderate. In a Della-Torre et al cohort, mean eosinophil count was 463 cells/μL (range: 20–2000). For patients with eosinophilia (27%), mean was 1062 cells/μL (range 600–2000).19 Culver et al reported eosinophilia in 38% of IgG4-RD (median: 0.44 × 109/L; range: 0.0–5.05) and 9% of HC (median: 0.16 × 109/L; range: 0.05–0.78) (p = 0.0042)4 Patients with hypereosinophilia (>1,5 × 109/L) are rare. In a cohort of 87 patients, Zhang et al identified only 3% of hypereosinophilia.41 In AIP, eosinophilia was reported in 11% (>600 cells/μL),3316% and 28% (>500 cells/μL).34,35 Eosinophilia is also more frequent in AIP compared to HC (12 vs 0% p = 0.0004).34 In tubulointerstitial nephritis, it is observed in 48% of patients.64 Cases of overlapping hypereosinophilic syndrome and IgG4-RD have also been reported. Moussiegt et al reported 44 cases among the French cohort.66
Eosinophils are also present in tissue of patient with IgG4-RD.1 Culver et al identified eosinophils infiltration in 86% (32 of 37) of specimens. It was higher than 10 eosinophils/high power field in 73%.4 In AIP, Sah et al also demonstrated moderate to severe eosinophilic infiltrates 13 patients (54%).34 They are also present in 48% of inflamed glands of patients with sialadenitis.57
In addition to allergies, eosinophilia is also more frequent in patient with DS. Wang et al demonstrated 32% of eosinophilia in DS, 41% in DS with other organ involvement and 17% in the group without DS (p = 0.048).30 In IgG4-ROD, eosinophilia was also found in 28%, but difference compared to subjects without ophthalmic disease was not statistically significant.26 There is also no difference between patients with or without pulmonary involvement.63
Patients with and without eosinophilia differs. In a large cohort of 425 patients, Zhang et al demonstrated that patients with eosinophilia tend to be male (73.3% vs 57.1%, p = 0.007), to have longer disease duration (12 months vs. 10, p = 0.034), dacryoadenitis (61.9% vs 16.4%, p = 0.014), submandibular sialadenitis (65.9% vs 49.5%, p = 0.007), lymphadenopathy (55.3% vs 42.0%, p = 0.037), and skin rashes (7.4% vs 2.7%, p = 0.034). They were also likely to have higher IgE (408 vs 302, p = 0.01) and IgG4 (17.00 vs 6.50, p < 0.001). They also tend to have a lower remission rate, but it was not statistically significant [83.8% (160/191) vs. 72.9% (35/48), p = 0.096]. No difference regarding the incidence of allergy was identified in their cohort41 However, Sanders et al showed that eosinophilia was associated with allergic manifestation with an odd ratio of 3.27 (1.19–9.02).25 Chen et al also demonstrated that the percentage of eosinophils correlated with the number of organs involved.43 In AIP, Sah et al reported higher frequency of allergy 6/22 (27%) in patients with eosinophilia vs 6/56 (11%) in patients without, but it was not statistically significant. There were no differences between AIP profiles or difference regarding eosinophilic infiltration.34
Since IgG4, IgE and eosinophils are elevated in many patients with IgG4-RD, reflecting Th2 inflammation, many looked at correlation between those parameters. Most studies showed significant positive correlation between serum level of IgE and IgG4.4,18,19,43,46,67 This finding is not surprising since it is also found in normal population.68 However, 3 others did not identify correlation.23,44,54 Studies specifically on IgG-RDS55 and AIP52 also did not. Regarding the correlation between eosinophils and IgG4, most studies demonstrated a positive correlation,19,41,43,46 including IgG4-RDS cohort.55 Only 1 study did not.23 This phenomenon is surprising and possibly inherent to Ig4-RD pathology. Indeed, in normal subjects, there is no correlation between IgG4 and eosinophils.68 Four studies also showed positive correlation between serum IgE and eosinophils count.4,18,41,67 It was not reproduced in 2 others, including an IgG4-RDS cohort.19,55
Allergy as a risk factors of relapse
Allergy and elevation of IgE, eosinophils, and IgG4 have been identified as risk factors of relapse in IgG4-RD. Allergy itself was identified by Liu et al and Peng et al as a risk factor of relapse (OR 3.058 95% CI 2.039–4.587; p < 0.001)40 (OR 2.647 95% CI 1.205–5.816; p < 0.015),45 but it was not reproduced in other studies.18,23,42 Elevated eosinophils, IgE and IgG4 were also found to be risk factors of relapse in a cohort of 60 biopsy proven IgG4-RD treated with rituximab by Wallace and al: absolute eosinophil levels (HR = 5.5, 95% CI: 1.4, 22.0) and IgE (HR = 6.9, 95% CI: 1.4, 33).65 Four other studies also confirmed eosinophils as a risk factor for relapse in IgG4-RD,18,27,42,45 but 2 did not.23,40 Association between high total serum IgE and relapse was found in 3 studies.4,18,52 In a large cohort of 435 patients, Zhou et al identified more relapse in patients with elevated baseline IgE >60 KU/L (16,2% vs 29,0%; p = 0.039), but follow-up IgE levels could not predict relapse. Hazard ratio for relapse with IgE levels of >125 KU/L was 1.894 (95% CI 1.022–3.508; p = 0.042). Moreover, in patients with elevated IgE, lacrimal gland, submandibular gland, and kidney were also associated with relapse.18 DS was also associated with relapse in 2 studies.40,42 Results regarding elevated IgG4 are discording, Zhou et al also reported higher risk of relapse as did Wallace et al, but it was not supported by others.23 In a study specifically on IgG4-ROD, allergy, eosinophils, IgE and IgG4 were not risk factors of relapse.26
Allergy/atopy as a cause of IgG4-related disease
To date, no allergens were identified as a cause of IgG4-RD. No common culprit could be identified with sIgE and/or skin prick testing and no temporal link with the beginning of allergies and IgG4-RD symptoms. Sanders et al reported that the allergies predated the onset of IgG4-RD by many years, also IgG4-RD did not impact allergic symptoms.25 Baqir et al also reported that the timing of asthma did not correlate with the beginning of IgG4-RD.69 Since chronic allergen exposure shifts the production of IgE to IgG4 (modified Th2 hypothesis), we could wonder if AIT could promote IgG4-RD. Della-Torre et al found no pathogenic link between AIT and IgG4-RD even if in their cohort AIT was used more frequently in patients with IgG4-RD (5%) than in the general Italian population (less than 2%). Of 116 subjects, 6 received AIT for aeroallergen allergy. There was no difference in epidemiologic, clinical, and immunologic features.50 Culver et al demonstrated elevated IgG4 response to diverse antigens. IgG4 radioassay to egg, milk, peanut, banana, rice, wheat, and cat were performed on 24 patients. Titers of most specific IgG4 antigens were higher in patients with IgG4-RD than in HC. This finding is not surprising given the polyclonal increase of serum IgG4 in IgG4-RD patients. There was no correlation between serum IgE and specific IgG4.24 However, this study was small, and the selection process of the allergens tested was not explained. Moreover, the diagnostic value of specific IgG4 is unknown. A WAO position paper does not recommend specific IgG4 testing for the identification of allergens.70 In a study on a Spanish adult population (n = 469), IgG4 were higher in male and atopic adults. Asymptomatic patients with positive skin test/elevated IgE were those with higher IgG4 levels, reinforcing the neutralizing role of IgG4 antibody in normal population. Five individuals had IgG4 levels above 135 mg/dL in this cohort, after a median of 11 years of follow-up, no case of IgG4-RD was identified.68
IgG4-RD, a Th2 disease
IgG4-RD is rather a TH2-inflammatory disease than an allergic disease. Classical Th2 cytokines IL-4,22,71, 72, 73 IL-5,71,72 and IL-1371 are elevated in patients’ serum. They are known to contribute to B cell proliferation and isotype class switching to IgE. IL-5 induces maturation and proliferation of eosinophils.74 IL-10, that is additionally produced by TH2 cells and T regulator cells, is also elevated in IgG4-RD.71, 72, 73 Recently, thymus and activation-regulated chemokine (TARC), which is elevated in patients with atopic dermatitis and asthma, was shown to also be elevated in patients with IgG4-RD.72,75,76 TARC ligates to CCR4 and CCR8 on Th2 cells and stimulates Th2 dominant inflammatory response.77 Umeda et al demonstrated that TARC was higher in IgG4-RD compared to SS and HC, particularly if lung involvement was present. It correlated with IgG4-RD RI score and number of organs involved. They also proved that TARC induces the formation of plasmablasts in patients with IgG4-RD independently of allergic status; however, this was not observed in HC.48
Interestingly, in 2014, in a cohort of 18 atopic vs 21 non-atopic IgG4-RD patients, Mattoo et al showed that IL-4, IL-5 and IL-13 were highly produced by CD4+GATA3+ cells (Th2) of atopic IgG4-RD patients, but not in non-atopic. They concluded that Th2 inflammation reflected an underlying allergic condition and was not a fundamental characteristic of IgG4-RD.20 Moreover, in 2016, Mattoo et al examined TCR-β repertoire of CD4+GATA3+ TH2 cells in 4 IgG4-RD subjects with atopy. The expansion was polyclonal in comparison to the oligoclonal expansion of CD4+SLAMF7+ CTL.21 However, other studies demonstrated that Th2 were higher in IgG4-RD subjects.22,78,79 Both Grados et al and Cargill et al demonstrated Th2 and TfH2 profile that were independent of the allergic/atopic status.22,79
T follicular helper cells (TfH), particularly Tfh2, are implicated in the pathogenesis of the disease. They are found in higher proportion in IgG4-RD,22,39,79,80 though some found an elevation of Tfh1.80,81 TfH2 cells correlates with IgG4,22,39,79, 80, 81 plasmablasts,22,39,79,81 and IL-4 levels.79,81,82 Chen et al also demonstrated a correlation with IgG4-RD RI and number of organs involved.80 Maehara et al showed that IL-4+BATF + Tfh were abundant around GC in IgG4-RD and could be linked to IgG4 class switching. BATF is a known transcription factor for IL-4 production in murine model. They correlated to serum IgG4 and plasmacytes in tissues. Those Tfh producers of IL-4 expressed high levels of Bcl6 and BATF but low GATA3. In transcriptomic studies, there was high levels of IL-4, but low level of IL-5 and IL-13.46 Moreover, Maehara et al showed that excessive production of IL-21 by TH2 cells in IgG4-RD induced expression of Bcl-6, which results in formation of multiple germinal centers.83 Chen et al also found high level of Bcl-6 and IL-21 in tissues.80 Additionally, Tsuboi et al demonstrated that IL-10, TGF-β, and AID were participating in IgG4 class switching.73 However, Akiyama et al showed that IL-4 and IL-21 enhanced the production of IgG4, but IL-4 did it preferentially in IgG4-RD and not in HC. Interestingly, IL-10 and IL-13 did not induce IgG4 production.82 Dupilumab, a monoclonal antibody that block IL-4 receptor alpha chain common to both IL-4 and IL-13 receptors, could therefore be a focus therapeutic option for IgG4-RD. Its efficacy has been proven in other Th2 pathology such as asthma and atopic dermatitis.84,85 However, to date, only 2 cases of IgG4-RD have been described. Simpson et al reported a patient successfully treated with dupilumab; however, the patients may have been also received corticosteroids.86 Ebbo et al also reported a patient that was successfully treated with maintenance dupilumab. It was associated with a reduction of total T helper 2 cells, T follicular helper (Tfh) cells, Tfh2 cells and plasmablasts. However, interestingly; although serum IgE significantly declined, serum IgG4 only slightly decreased.87
Mast cells and basophils in IgG4-RD
Mast cells and basophils could also be a source of TH2 cytokines. Culver et al identified IgE-positive cells (presumably mast cells) in 50% (4/8) of biopsies at >10/HPF. Mast cells are known to secrete Th2 cytokines88 and to stimulate fibrosis89 under chronic stimulation of FcεRI by elevated IgE without antigen stimulation.90 Hence, mast cell infiltration was found in IgG4-related fibrosclerotic mesenteric mass.4 Nishida et al also showed more internalization of IgE and FcεRI in IgG4-RD mast cells compared to non-specific lymphoid hyperplasia, which could reflect mast cells activation.91 Takeuchi et al also demonstrated that mast cells present in biopsies were producers of IL-4, IL-10, IL-13, and TGF-beta. They did not seem to be produced by T cells of T regulator cells (Treg). TGF-beta produced by mast cell could induce differentiation of Treg.92,93 Regarding basophils, a small study showed they induced IgG4 secretion (not IgE) by B cells via BAFF production upon stimulation by TLR2 and TLR4. IL-13 and T cells were not necessary.94 Basophils could also activate Th2 via IL-4 secretion although it remains controversial.74 Th2 inflammation could also be in part induced by M2 macrophages via production of IL-33 after stimulation of TLR7.95,96
In conclusion, the exact role of allergy/atopy in IgG4-RD pathogenesis remains unclear. Th2/Tfh2 profile seems to be inherent to IgG4-RD itself, as are elevation of IgE and eosinophils. Production of IgG4 is also secondary to polyclonal expansion IgG4-switched B cells rather than an oriented response as in Th2 modified hypothesis and AIT. Allergic rhinitis and asthma are frequent. Despite being present in a high proportion of patients, there is no clear causality link between allergy/atopy and IgG4-RD. No common allergen has been identified. However, allergic/atopic subjects show some particularities within IgG4-RD patients: possibly more head, neck, and thoracic involvement, higher IgE and eosinophils and less RPF. An immune dysfunction common to allergic/atopic patients could contribute to this phenotype. Moreover, patients with DS could represent a specific subgroup as they have higher prevalence of allergies, higher IgE and eosinophils, and higher rates of relapse. We could wonder if chronic allergen stimulation of ORL sphere is modifying the phenotype. However, many observations were made in small and retrospective studies. Allergy tests were rarely used to confirm diagnosis and were not analyzed considering symptomatology of patients. Larger studies with stronger allergy evaluations are needed to provide an accurate picture of the allergic population within IgG4-RD. The role of innate immune cells implicated in allergy, such as eosinophils, mast cells and basophils, should be investigated further. Also, specific TH2 cytokine targeted therapy, such as dupilumab,87 should be investigated further, as they could offer an option to conventional therapy of corticosteroids and rituximab that are not used without side effects.
Abbreviations
AIP, Autoimmune pancreatitis; AIT, Allergen immunotherapy; EAACI, Allergy definition of the European Academy of Allergy & Clinical Immunology; CRS, Chronic rhinosinusitis; DS, Dacryoadenitis and sialadenitis; HC, Healthy control; IgG4-RDS, IgG4 related DS; IgG4-RD, IgG4-related disease; IgG4-ROD, IgG4 related ophthalmic disease; PFT, Pulmonary functional test; RPF, Retroperitoneal fibrosis; TfH, T follicular helper cells; TARC, Thymus and activation-regulated chemokine; Treg, T regulator cells; SS, Sjögren Syndrome; sIgE, Specific IgE.
Acknowledgement
The authors acknowledge financial support from the Department of Pediatrics of University of Sherbrooke to cover publication fees.
Availability of data and materials
Not applicable.
Author contributions
KDG, ME, PC and NS had the idea for the article, KDG performed the literature search and data analysis and drafted the work, ME, PC and NS critically revised the work. All authors approved the final manuscript.
Ethics statement
This manuscript is a review. It did not involve human or animal subject.
Statements and declarations
All authors approved the final manuscript and agreed to publish the work in WAO Journal.
All authors agree with the Editorial policy. This work has not been published elsewhere.
Potential competing interests
The authors report no competing interests.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Stone J.H., Zen Y., Deshpande V. IgG4-related disease. N Engl J Med. Feb 9 2012;366(6):539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande V., Zen Y., Chan J.K., et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. Sept 2012;25(9):1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 3.Lang D., Zwerina J., Pieringer H. IgG4-related disease: current challenges and future prospects. Therapeut Clin Risk Manag. Feb 2016:189. doi: 10.2147/TCRM.S99985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culver E.L., Sadler R., Bateman A.C., et al. Increases in IgE, eosinophils, and mast cells can be used in diagnosis and to predict relapse of IgG4-related disease. Clin Gastroenterol Hepatol. Sept 2017;15(9):1444–1452.e6. doi: 10.1016/j.cgh.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daëron M., Jaeger S., Du Pasquier L., Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. Aug 2008;224(1):11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 6.Rispens T., den Bleker T.H., Aalberse R.C. Hybrid IgG4/IgG4 Fc antibodies form upon ‘Fab-arm’ exchange as demonstrated by SDS-PAGE or size-exclusion chromatography. Mol Immunol. Apr 2010;47(7-8):1592–1594. doi: 10.1016/j.molimm.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Young E., Lock E., Ward D.G., Cook A., Harding S., Wallis G.L.F. Estimation of polyclonal IgG4 hybrids in normal human serum. Immunology. July 2014;142(3):406–413. doi: 10.1111/imm.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeannin P., Lecoanet S., Delneste Y., Gauchat J.F., Bonnefoy J.Y. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol Baltim Md 1950. Apr 1998;160(7):3555–3561. [PubMed] [Google Scholar]
- 9.Wood N., Bourque K., Donaldson D.D., et al. IL-21 effects on human IgE production in response to IL-4 or IL-13. Cell Immunol. Sept 2004;231(1-2):133–145. doi: 10.1016/j.cellimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Platts-Mills T.A.E., Woodfolk J.A., Erwin E.A., Aalberse R. Mechanisms of tolerance to inhalant allergens: the relevance of a modified Th2 response to allergens from domestic animals. Springer Semin Immunopathol. Feb 2004;25(3-4):271–279. doi: 10.1007/s00281-003-0149-8. [DOI] [PubMed] [Google Scholar]
- 11.Platts-Mills T., Vaughan J., Squillace S., Woodfolk J., Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. March 2001;357(9258):752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 12.James L.K., Shamji M.H., Walker S.M., et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. Feb 2011;127(2):509–516.e5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 13.Francis J.N., James L.K., Paraskevopoulos G., et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. May 2008;121(5):1120–1125.e2. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 14.Vickery B.P., Lin J., Kulis M., et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. Jan 2013;131(1):128–134.e3. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcock L.K., Francis J.N., Durham S.R. IgE-facilitated antigen presentation: role in allergy and the influence of allergen immunotherapy. Immunol Allergy Clin. May 2006;26(2):333–347. doi: 10.1016/j.iac.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.James L.K., Till S.J. Potential mechanisms for IgG4 inhibition of immediate hypersensitivity reactions. Curr Allergy Asthma Rep. March 2016;16(3):23. doi: 10.1007/s11882-016-0600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson S.G.O., Bieber T., Dahl R., et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the World allergy organization, October 2003. J Allergy Clin Immunol. May 2004;113(5):832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J., Peng Y., Peng L., et al. Serum IgE in the clinical features and disease outcomes of IgG4-related disease: a large retrospective cohort study. Arthritis Res Ther. 2020;22(1) doi: 10.1186/s13075-020-02338-1. 255-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Della Torre E., Mattoo H., Mahajan V.S., Carruthers M., Pillai S., Stone J.H. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy. Feb 2014;69(2):269–272. doi: 10.1111/all.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattoo H., Della-Torre E., Mahajan V.S., Stone J.H., Pillai S. Circulating Th2 memory cells in IgG4-related disease are restricted to a defined subset of subjects with atopy. Allergy. March 2014;69(3):399–402. doi: 10.1111/all.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattoo H., Mahajan V.S., Maehara T., et al. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol. 2016;138(3):825–838. doi: 10.1016/j.jaci.2015.12.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grados A., Ebbo M., Piperoglou C., et al. T cell polarization toward T(H)2/T(FH)2 and T(H)17/T(FH)17 in patients with IgG4-related disease. Front Immunol. 2017;8:235. doi: 10.3389/fimmu.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campochiaro C., Ramirez G., Bozzolo E., et al. IgG4-related disease in Italy: clinical features and outcomes of a large cohort of patients. Scand J Rheumatol. March 2016;45(2):135–145. doi: 10.3109/03009742.2015.1055796. [DOI] [PubMed] [Google Scholar]
- 24.Culver E.L., Vermeulen E., Makuch M., et al. Increased IgG4 responses to multiple food and animal antigens indicate a polyclonal expansion and differentiation of pre-existing B cells in IgG4-related disease. Ann Rheum Dis. May 2015;74(5):944–947. doi: 10.1136/annrheumdis-2014-206405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders S., Fu X., Zhang Y., et al. Lifetime allergy symptoms in IgG4-related disease: a case-control study. Arthritis Care Res. 2022 Jul;74(7):1188–1195. doi: 10.1002/acr.24545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Z., Mou D., Wang Z., et al. Clinical features and relapse risks of IgG4-related ophthalmic disease: a single-center experience in China. Arthritis Res Ther. Dec 2021;23(1):98. doi: 10.1186/s13075-021-02489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H., Teng F., Zhang P., et al. Differences in clinical characteristics of IgG4-related disease across age groups: a prospective study of 737 patients. Rheumatology. June 2021;60(6):2635–2646. doi: 10.1093/rheumatology/keaa651. [DOI] [PubMed] [Google Scholar]
- 28.Zen Y., Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol. Dec 2010;34(12):1812–1819. doi: 10.1097/PAS.0b013e3181f7266b. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X., Zhang P., Zhang W. Clinical significance of allergy in IgG4-related disease [abstract] Arthritis Rheumatol [Internet] 2018 https://acrabstracts.org/abstract/clinical-significance-of-allergy-in-igg4-related-disease/ [cited March 2021]; Available on: [Google Scholar]
- 30.Wang M., Zhang P., Lin W., et al. Differences and similarities between IgG4-related disease with and without dacryoadenitis and sialoadenitis: clinical manifestations and treatment efficacy. Arthritis Res Ther. Feb 2019;21(1):44. doi: 10.1186/s13075-019-1828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Xue M., Wang Z., et al. Salivary gland involvement disparities in clinical characteristics of IgG4-related disease: a retrospective study of 428 patients. Rheumatology. Aug 2019:kez280. doi: 10.1093/rheumatology/kez280. [DOI] [PubMed] [Google Scholar]
- 32.Fei Y., Shi J., Lin W., et al. Intrathoracic involvements of immunoglobulin G4-related sclerosing disease. Medicine (Baltimore) 2015;94(50) doi: 10.1097/MD.0000000000002150. e2150-e2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamisawa T., Anjiki H., Egawa N., Kubota N. Allergic manifestations in autoimmune pancreatitis. Eur J Gastroenterol Hepatol. Oct 2009;21(10):1136–1139. doi: 10.1097/meg.0b013e3283297417. [DOI] [PubMed] [Google Scholar]
- 34.Sah R.P., Pannala R., Zhang L., Graham R.P., Sugumar A., Chari S.T. Eosinophilia and allergic disorders in autoimmune pancreatitis. Am J Gastroenterol. Nov 2010;105(11):2485–2491. doi: 10.1038/ajg.2010.236. [DOI] [PubMed] [Google Scholar]
- 35.Kuruma S., Kamisawa T., Tabata T., et al. Allergen-specific IgE antibody serologic assays in patients with autoimmune pancreatitis. Intern Med. 2014;53(6):541–543. doi: 10.2169/internalmedicine.53.0963. [DOI] [PubMed] [Google Scholar]
- 36.Saeki T., Kobayashi D., Ito T., Tamura M., Yoshikawa S., Yamazaki H. Comparison of clinical and laboratory features of patients with and without allergic conditions in IgG4-related disease: a single-center experience in Japan. Mod Rheumatol. Sept 2018;28(5):845–848. doi: 10.1080/14397595.2017.1416891. [DOI] [PubMed] [Google Scholar]
- 37.Takano K., Abe A., Yajima R., et al. Clinical evaluation of sinonasal lesions in patients with immunoglobulin G4-related disease. Ann Otol Rhinol Laryngol. Dec 2015;124(12):965–971. doi: 10.1177/0003489415593557. [DOI] [PubMed] [Google Scholar]
- 38.Ebbo M., Daniel L., Pavic M., et al. IgG4-Related systemic disease: features and treatment response in a French cohort results of a multicenter registry. Medicine (Baltimore) Jan 2012;91(1):49–56. doi: 10.1097/MD.0b013e3182433d77. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama M., Yasuoka H., Yamaoka K., et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther. July 2016;18:167. doi: 10.1186/s13075-016-1064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Zeng Q., Zhu L., et al. Relapse predictors and serologically unstable condition of IgG4-related disease: a large Chinese cohort. Rheumatology. August 2020;59(8):2115–2123. doi: 10.1093/rheumatology/kez669. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X., Zhang P., Li J., et al. Different clinical patterns of IgG4-RD patients with and without eosinophilia. Sci Rep. Nov 2019;9(1) doi: 10.1038/s41598-019-52847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L., Zhang P., Wang M., et al. Failure of remission induction by glucocorticoids alone or in combination with immunosuppressive agents in IgG4-related disease: a prospective study of 215 patients. Arthritis Res Ther. Dec 2018;20(1):65. doi: 10.1186/s13075-018-1567-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y., Zhao J.-Z., Feng R.-E., et al. Types of organ involvement in patients with immunoglobulin G4-related disease. Chin Med J (Engl) July 2016;129(13):1525–1532. doi: 10.4103/0366-6999.184459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin W., Lu S., Chen H., et al. Clinical characteristics of immunoglobulin G4–related disease: a prospective study of 118 Chinese patients. Rheumatology. Nov 2015;54(11):1982–1990. doi: 10.1093/rheumatology/kev203. [DOI] [PubMed] [Google Scholar]
- 45.Peng Y., Li J.Q., Zhang P.P., et al. Clinical outcomes and predictive relapse factors of IgG4-related disease following treatment: a long-term cohort study. J Intern Med. Nov 2019;286(5):542–552. doi: 10.1111/joim.12942. [DOI] [PubMed] [Google Scholar]
- 46.Maehara T., Mattoo H., Mahajan V.S., et al. The expansion in lymphoid organs of IL-4(+) BATF(+) T follicular helper cells is linked to IgG4 class switching in vivo. Life Sci Alliance. Jan 2018;1(1) doi: 10.26508/lsa.201800050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanders S., Della Torre E., Perugino C.A., et al. Salivary gland disease in IgG4-related disease is associated with allergic histories [abstract] Arthritis Rheumatol. 2018;70(suppl 10) https://acrabstracts.org/abstract/salivary-gland-disease-in-igg4-related-disease-is-associated-with-allergic-histories/ [Google Scholar]
- 48.Umeda M., Origuchi T., Kawashiri S.Y., et al. Thymus and activation-regulated chemokine as a biomarker for IgG4-related disease. Sci Rep. Apr 2020;10(1):6010. doi: 10.1038/s41598-020-62941-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y., Zheng M., Cui L., et al. IgG4-related disease: association between chronic rhino-sinusitis and systemic symptoms. Eur Arch Oto-Rhino-Laryngol. Aug 2018;275(8):2013–2019. doi: 10.1007/s00405-018-5013-5. [DOI] [PubMed] [Google Scholar]
- 50.Della-Torre E., Germanò T., Ramirez G.A., Dagna L., Yacoub M.R. IgG4-related disease and allergen-specific immunotherapy. Ann Allergy Asthma Immunol. June 2020;124(6):631–633. doi: 10.1016/j.anai.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 51.Lanzillotta M., Campochiaro C., Mancuso G., et al. Clinical phenotypes of IgG4-related disease reflect different prognostic outcomes. Rheumatology. 2020;59(9):2435–2442. doi: 10.1093/rheumatology/keaa221. 1 Sept. [DOI] [PubMed] [Google Scholar]
- 52.Hirano K. Clinical analysis of high serum IgE in autoimmune pancreatitis. World J Gastroenterol. 2010;16(41):5241. doi: 10.3748/wjg.v16.i41.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue D., Yoshida K., Yoneda N., et al. IgG4-Related disease: dataset of 235 consecutive patients. Medicine (Baltimore) Apr 2015;94(15) doi: 10.1097/MD.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsui S., Okazawa S., Tokui K., et al. Allergy in IgG4-related disease. J Allergy Clin Immunol. 2018;141(2) 1 Feb. [Google Scholar]
- 55.Yamamoto M., Takano K., Kamekura R., et al. Analysis of allergic reaction in IgG4-related disease. Mod Rheumatol. Nov 2019;29(6):1063–1065. doi: 10.1080/14397595.2019.1572488. [DOI] [PubMed] [Google Scholar]
- 56.Masaki Y., Dong L., Kurose N., et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. August. 2009;68(8):1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 57.Li W., Chen Y., Sun Z.-P., et al. Clinicopathological characteristics of immunoglobulin G4-related sialadenitis. Arthritis Res Ther. Dec 2015;17(1):186. doi: 10.1186/s13075-015-0698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pawankar R., Holgate S.T., Canonica W., Lockey R.F., Blaiss M.S. 2013. WAO White Book on Allergy. Update. [Google Scholar]
- 59.Nathan R.A., Meltzer E.O., Derebery J., et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. Nov 2008;29(6):600–608. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 60.Moteki H., Yasuo M., Hamano H., Uehara T., Usami S. IgG4-related chronic rhinosinusitis: a new clinical entity of nasal disease. Acta Otolaryngol (Stockh) May 2011;131(5):518–526. doi: 10.3109/00016489.2010.533699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piao Y., Wang C., Yu W., et al. Concomitant occurrence of Mikulicz's disease and immunoglobulin G4-related chronic rhinosinusitis: a clinicopathological study of 12 cases. Histopathology. March 2016;68(4):502–512. doi: 10.1111/his.12775. [DOI] [PubMed] [Google Scholar]
- 62.Bernstein I.L., Li J.T., Bernstein D.I., et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. March 2008;100(3):S1–S148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- 63.Cao L., Chen Y.-B., Zhao D.-H., Shi W.-F., Meng S., Xie L.-X. Pulmonary function tests findings and their diagnostic value in patients with IgG4-related disease. J Thorac Dis. March 2017;9(3):547–554. doi: 10.21037/jtd.2017.02.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saeki T., Nishi S., Imai N., et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. Nov 2010;78(10):1016–1023. doi: 10.1038/ki.2010.271. [DOI] [PubMed] [Google Scholar]
- 65.Wallace Z.S., Mattoo H., Mahajan V.S., et al. Predictors of disease relapse in IgG4-related disease following rituximab. Rheumatol Oxf. June 2016;55(6):1000–1008. doi: 10.1093/rheumatology/kev438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moussiegt A., Müller R., Ebbo M., et al. IgG4-related disease and hypereosinophilic syndrome: overlapping phenotypes. Autoimmun Rev. Sept 2021;20(9) doi: 10.1016/j.autrev.2021.102889. [DOI] [PubMed] [Google Scholar]
- 67.Li J., Peng Y., Zhang Y., et al. Identifying clinical subgroups in IgG4-related disease patients using cluster analysis and IgG4-RD composite score. Arthritis Res Ther. Jan 2020;22(1):7. doi: 10.1186/s13075-019-2090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carballo I., Alvela L., Pérez L.F., et al. Serum concentrations of IgG4 in the Spanish adult population: relationship with age, gender, and atopy. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baqir M., Garrity J.A., Vassallo R., Witzig T.E., Ryu J.H. Asthma and orbital immunoglobulin G4–related disease. Ann Allergy Asthma Immunol. Apr 2016;116(4):313–316. doi: 10.1016/j.anai.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Ansotegui I.J., Melioli G., Canonica G.W., et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. Feb 2020;13(2) doi: 10.1016/j.waojou.2019.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zen Y., Fujii T., Harada K., et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. June 2007;45(6):1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka A., Moriyama M., Nakashima H., et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. Jan 2012;64(1):254–263. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 73.Tsuboi H., Matsuo N., Iizuka M., et al. Analysis of IgG4 class switch-related molecules in IgG4-related disease. Arthritis Res Ther. 2012;14(4) doi: 10.1186/ar3924. R171-R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker J.A., McKenzie A.N.J. TH2 cell development and function. Nat Rev Immunol. Feb 2018;18(2):121–133. doi: 10.1038/nri.2017.118. [DOI] [PubMed] [Google Scholar]
- 75.Sekiya T., Yamada H., Yamaguchi M., et al. Increased levels of a TH2-type CC chemokine thymus and activation-regulated chemokine (TARC) in serum and induced sputum of asthmatics. Allergy. Feb 2002;57(2):173–177. doi: 10.1034/j.1398-9995.2002.5720256.x. [DOI] [PubMed] [Google Scholar]
- 76.Kataoka Y. Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. J Dermatol. March 2014;41(3):221–229. doi: 10.1111/1346-8138.12440. [DOI] [PubMed] [Google Scholar]
- 77.Saeki H., Tamaki K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J Dermatol Sci. Aug 2006;43(2):75–84. doi: 10.1016/j.jdermsci.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Heeringa J.J., Karim A.F., van Laar J.A.M., et al. Expansion of blood IgG(4)(+) B, T(H)2, and regulatory T cells in patients with IgG(4)-related disease. J Allergy Clin Immunol. May 2018;141(5):1831–1843.e10. doi: 10.1016/j.jaci.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 79.Cargill T., Makuch M., Sadler R., et al. Activated T-follicular helper 2 cells are associated with disease activity in IgG4-related sclerosing cholangitis and pancreatitis. Clin Transl Gastroenterol. 2019;10(4) doi: 10.14309/ctg.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y., Lin W., Yang H., et al. Aberrant expansion and function of follicular helper T cell subsets in IgG4-related disease. Arthritis Rheumatol. Nov 2018;70(11):1853–1865. doi: 10.1002/art.40556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akiyama M., Suzuki K., Yamaoka K., et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol. Sept 2015;67(9):2476–2481. doi: 10.1002/art.39209. [DOI] [PubMed] [Google Scholar]
- 82.Akiyama M., Yasuoka H., Yoshimoto K., Takeuchi T. Interleukin-4 contributes to the shift of balance of IgG subclasses toward IgG4 in IgG4-related disease. Cytokine. Oct 2018;110:416–419. doi: 10.1016/j.cyto.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Maehara T., Moriyama M., Nakashima H., et al. Interleukin-21 contributes to germinal centre formation and immunoglobulin G4 production in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Ann Rheum Dis. Dec 2012;71(12):2011–2019. doi: 10.1136/annrheumdis-2012-201477. [DOI] [PubMed] [Google Scholar]
- 84.Agache I., Song Y., Rocha C., et al. Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines—recommendations on the use of biologicals in severe asthma. Allergy. May 2020;75(5):1058–1068. doi: 10.1111/all.14268. [DOI] [PubMed] [Google Scholar]
- 85.Frampton J.E., Blair H.A. Dupilumab: a review in moderate-to-severe atopic dermatitis. Am J Clin Dermatol. Aug 2018;19(4):617–624. doi: 10.1007/s40257-018-0370-9. [DOI] [PubMed] [Google Scholar]
- 86.Simpson R.S., Lau S.K.C., Lee J.K. Dupilumab as a novel steroid-sparing treatment for IgG4-related disease. Ann Rheum Dis. Apr 2020;79(4):549–550. doi: 10.1136/annrheumdis-2019-216368. [DOI] [PubMed] [Google Scholar]
- 87.Ebbo M., De Sainte-Marie B., Muller R., et al. Comment on article: ‘Dupilumab as a novel steroid-sparing treatment for IgG 4 -related disease’ by Simpson et al. Ann Rheum Dis. Jan 2020 doi: 10.1136/annrheumdis-2020-217010. annrheumdis-2020-217010. [DOI] [PubMed] [Google Scholar]
- 88.Kashiwakura J., Kawakami Y., Yuki K., et al. Polyclonal IgE induces mast cell survival and cytokine production. Allergol Int. 2009;58(3):411–419. doi: 10.2332/allergolint.08-OA-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mukai K., Tsai M., Saito H., Galli S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. March 2018;282(1):121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalesnikoff J., Huber M., Lam V., et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. June 2001;14(6):801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 91.Nishida K., Gion Y., Takeuchi M., et al. Mast cells exhibiting strong cytoplasmic staining for IgE and high affinity IgE receptor are increased in IgG4-related disease. Sci Rep. March 2018;8(1):4656. doi: 10.1038/s41598-018-23043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeuchi M., Sato Y., Ohno K., et al. T helper 2 and regulatory T-cell cytokine production by mast cells: a key factor in the pathogenesis of IgG4-related disease. Mod Pathol. Aug 2014;27(8):1126–1136. doi: 10.1038/modpathol.2013.236. [DOI] [PubMed] [Google Scholar]
- 93.Takeuchi M., Ohno K., Takata K., et al. Interleukin 13-positive mast cells are increased in immunoglobulin G4-related sialadenitis. Sci Rep. Jan 2015;5:7696. doi: 10.1038/srep07696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watanabe T., Yamashita K., Sakurai T., et al. Toll-like receptor activation in basophils contributes to the development of IgG4-related disease. J Gastroenterol. Feb 2013;48(2):247–253. doi: 10.1007/s00535-012-0626-8. [DOI] [PubMed] [Google Scholar]
- 95.Ishiguro N., Moriyama M., Furusho K., et al. Activated M2 macrophages contribute to the pathogenesis of IgG4-related disease via toll-like receptor 7/interleukin-33 signaling. Arthritis Rheumatol. Jan 2020;72(1):166–178. doi: 10.1002/art.41052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Furukawa S., Moriyama M., Miyake K., et al. Interleukin-33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4-related disease. Sci Rep. Feb 2017;7 doi: 10.1038/srep42413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.