Key Points
Question
Is there a heritable, traitlike component of subjective memory concern that would confound its use as a state-related indicator of dementia risk?
Findings
This cohort study of 1555 adults who completed at least 1 follow-up assessment found evidence for the heritability of subjective memory concern, which was associated with genetic risk indicators of Alzheimer disease and anxiety and remained relatively stable across 3 decades.
Meaning
The findings in this study suggest that assessment of subjective memory concern will need to develop ways to distinguish longstanding memory concerns from concerns that reflect recent memory changes so as to more accurately measure the more relevant statelike component for the improvement of dementia risk evaluation.
This cohort study evaluates the heritability of subjective memory concern and its association with certain genetic risk indicators.
Abstract
Importance
Subjective memory concern has long been considered a state-related indicator of impending cognitive decline or dementia. The possibility that subjective memory concern may itself be a heritable trait is largely ignored, yet such an association would substantially confound its use in clinical or research settings.
Objective
To assess the heritability and traitlike dimensions of subjective memory concern and its clinical correlates.
Design, Setting, and Participants
This longitudinal twin cohort study was conducted from 1967 to 2019 among male adults with a mean (SD) age of 37.75 (2.52) years to follow-up at mean ages of 56.15 (2.72), 61.50 (2.43), and 67.35 (2.57) years (hereafter, 38, 56, 62, and 67 years, respectively) in the Vietnam Era Twin Study of Aging. The study included a national community-dwelling sample with health, education, and lifestyle characteristics comparable to a general sample of US men in this age cohort. Participants were monozygotic and dizygotic twins randomly recruited from the Vietnam Era Twin Registry. Data were analyzed from May 2021 to December 2022.
Main Outcomes and Measures
Measures included subjective memory concern at 4 time points; objective memory, depressive symptoms, and anxiety at the last 3 time points; negative emotionality (trait neuroticism) at age 56 years; polygenic risk scores (PRSs) for neuroticism, depression, and Alzheimer disease; APOE genotype; and parental history of dementia. Primary outcomes were heritability and correlations between subjective memory concern and other measures.
Results
The sample included 1555 male adults examined at age 38 years, 520 at age 56 years (due to late introduction of subjective memory concern questions), 1199 at age 62 years, and 1192 at age 67 years. Phenotypically, subjective memory concerns were relatively stable over time. At age 56 years, subjective memory concern had larger correlations with depressive symptoms (r, 0.32; 95% CI, 0.21 to 0.42), anxiety (r, 0.36; 95% CI, 0.18 to 0.51), and neuroticism (r, 0.34; 95% CI, 0.26 to 0.41) than with objective memory (r, −0.24; 95% CI, −0.33 to −0.13). Phenotypic results were similar at ages 62 and 67 years. A best-fitting autoregressive twin model indicated that genetic influences on subjective memory concern accumulated and persisted over time (h2 = 0.26-0.34 from age 38-67 years). At age 56 years, genetic influences for subjective memory concern were moderately correlated with genetic influences for anxiety (r, 0.36; 95% CI, 0.18 to 0.51), negative emotionality (r, 0.51; 95% CI, 0.44-0.57), and depressive symptoms (r, 0.20; 95% CI, 0.10 to 0.29) as well as objective memory (r, −0.22; 95% CI, −0.30 to −0.14). Similar genetic correlations were seen at ages 62 and 67 years. The neuroticism PRS was associated with subjective memory concern at age 38 years (r, 0.10; 95% CI, 0.03. to 0.18) and age 67 years (r, 0.09; 95% CI, 0.01 to 0.16). Subjective memory concern was not associated with any Alzheimer disease risk measures.
Conclusions and Relevance
This cohort study found stable genetic influences underlying subjective memory concern dating back to age 38 years. Subjective memory concern had larger correlations with affect-related measures than with memory-related measures. Improving the utility of subjective memory concern as an indicator of impending cognitive decline and dementia may depend on isolating its statelike component from its traitlike component.
Introduction
Approximately 20% of adults report subjective memory concern involving self-reported difficulties and worries about learning and recall.1 Subjective memory concern is of considerable interest due to its association with poorer quality of life,2 poorer mental health,3 and elevated risk of Alzheimer disease and related dementias (ADRD).2,3,4,5,6 Originally, researchers hypothesized that subjective memory concern was state related, capturing new memory decline as a person aged.7 However, subjective memory concern is only weakly associated with actual memory performance,8,9 and stronger associations have been found between subjective memory concern and depressive symptoms.10 Regarding dementia, subjective memory concern is viewed primarily as an indicator of impending cognitive decline preceding dementia, as indicated by the international workgroup that defined the diagnostic term subjective cognitive decline.1 In potential contradiction with subjective memory concern as a marker of imminent decline, a small longitudinal study found stability of subjective memory concern (r, 0.58; n = 67) assessed at ages 40 and 70 years.11 This level of stability across 30 years suggests a meaningful traitlike component of subjective memory concern—that is, some individuals may be characterologically disposed to worry more or less about their memory. Thus, subjective memory concern in older adults—most often assessed as a state-related indicator of impending cognitive decline—would be partially confounded by any traitlike components.
In the UK Twin Register,12 heritability of subjective memory concern ranged from 0.37 to 0.64 in 3886 adults aged 20 to 84 years. In the Older Australian Twins Study,13 heritability of subjective memory concern was 0.60 among 268 participants. In contrast, newly occurring subjective cognitive decline was not found to be heritable in 11 926 Swedish Twin Registry14 participants aged 65 years and older. The latter finding suggests a statelike phenomenon, possibly signaling impending dementia-related cognitive decline that we would not expect in younger adults. On the other hand, considerable subjective memory concern heritability must indicate something about specific traits. Although subjective memory concern may be heritable, we still know little about the extent to which the same or different genetic factors may influence subjective memory concern at different ages or the extent to which shared genetic influences underlie its association with depressive symptoms, anxiety, ADRD, or objective memory performance.
We examined genetic and environmental factors associated with subjective memory concern at 4 time points over 30 years among adult male individuals with a mean age range of 38 to 67 years. We used twin models to test for the presence of common and new genetic influences over time. We expected subjective memory concern to be relatively stable as people aged, and we hypothesized that the stable variation would be primarily associated with genetic factors. We hypothesized that subjective memory concern would have larger correlations with depressive symptoms and anxiety than objective memory performance3,10,15,16,17 and that shared genetic factors would partially underlie the association with depressive symptoms and anxiety. To assess construct validity, we examined associations of subjective memory concern with polygenic risk scores (PRSs) for depression, neuroticism, AD, negative affect, APOE genotype, and parental history of dementia.
Methods
Participants
The Vietnam Era Twin Study of Aging (VETSA) is a longitudinal study of cognitive and brain aging and risk for AD in community-dwelling male twins, both of whom served in the US military during the period of US involvement in the Vietnam War (1965 to 1975), conducted from 1967 to 2019.18,19,20 Participants were randomly recruited from a Vietnam Era Twin Registry study.21 The Vietnam Era Twin Registry only included male twins as there were too few female twins who both served during the Vietnam war. VETSA inclusion criteria were being in the selected age range and both twins in a pair agreeing to participate in the baseline assessment. About 20% of respondents reported combat exposure. Participants were comparable to the general US population in their age cohort in terms of health, education, and lifestyle factors.22
VETSA methods have been described in detail elsewhere.18,19,20 Wave 1 data collection took place from 2003 to 2007, when participants were aged 51 to 61 years. Wave 2 took place between 2009 and 2013, when participants were aged 56 to 67 years, and wave 3 took place between 2016 to 2019, when participants were aged 61 to 73 years. In the 3 study waves, mean (SD) ages were 56.15 (2.72; n = 1237), 61.50 (2.45; n = 1199), and 67.35 (2.53; n = 1192) years (hereafter referred to as ages 56, 62, and 67 years, respectively). Sample size was lower at wave 1 (n = 520) for these analyses because subjective memory concern questions were added partway into data collection. Overall, 1555 participants provided data for at least 1 wave, providing more than 80% power to detect small phenotypic effect sizes (r, 0.10; d, 0.20) for bivariate correlations (required n = 779; G*Power23) and the planned structural equation modeling (required n = 1258; Westland algorithm24) as well as 80% power in univariate biometric twin models to detect a heritability of 0.25 (umx package in R version 4.0.2 [R Foundation]). Power is greater for multivariate twin analysis. For ease of presentation, we used mean ages to refer to specific assessment waves. Sample characteristics are shown in Table 1 and eTable 1 in Supplement 1. Participants who were missing data on subjective memory concern were not different on demographic or baseline study variables shown in Table 1. Procedures were approved by the institutional review boards at the University of California, San Diego, and Boston University, Boston, Massachusetts. Participants provided written informed consent. Results are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Data were analyzed from May 2021 to December 2022.
Table 1. Descriptives of Analytical Sample Across the Study Waves.
| Variable | Age 38 y assessment | Vietnam Era Twin Study of Aging | ||
|---|---|---|---|---|
| Wave 1 | Wave 2 | Wave 3 | ||
| Sample size, No. | 1555 | 520 | 1199 | 1192 |
| Monozygotic pairs, No. | 436 | 134 | 328 | 301 |
| Dizygotic pairs, No. | 285 | 116 | 233 | 206 |
| Unpaired twins, No. | 113 | 30 | 77 | 344 |
| Age, mean (SD), y | 37.75 (2.52) | 56.15 (2.72) | 61.50 (2.43) | 67.35 (2.57) |
| Race, No.a | ||||
| American Indian or Alaska Native | 4 | 2 | 2 | 5 |
| Asian | 0 | 0 | 0 | 0 |
| Black | 62 | 24 | 55 | 72 |
| Native Hawaiian or Other Pacific Islander | 2 | 2 | 0 | 9 |
| White | 1046 | 326 | 880 | 1064 |
| More than 1 race | 18 | 6 | 18 | 16 |
| Ethnicity, No.a | ||||
| Hispanic or Latino | 30 | 3 | 24 | 31 |
| Not Hispanic or Latino | 1513 | 511 | 1170 | 1161 |
| Subjective memory concernb | ||||
| Monozygotic correlation, r (95% CI) | 0.23 (0.18 to 0.28) | 0.34 (0.26 to 0.41) | 0.29 (0.23 to 0.34) | 0.38 (0.24 to 0.34) |
| Dizygotic correlation, r (95% CI) | 0.11 (0.06 to 0.16) | 0.12 (0.03 to 0.20) | 0.11 (0.05 to 0.17) | 0.05 (−0.01 to 0.11) |
| Trouble with memory,c mean (SD) | 1.83 (0.97) | NA | NA | NA |
| Rating of memory problem,d mean (SD) | NA | 1.98 (0.65) | 1.81 (0.66) | 1.86 (0.66) |
| Concern about memory,e mean (SD) | NA | 2.10 (0.84) | 2.00 (0.85) | 2.08 (0.85) |
Abbreviation: NA, not applicable.
Race and ethnicity were self-reported based on categories provided by the investigators and were considered for this study to adjust for possible sociocultural differences in rating subjective memory concern.
Subjective memory concern is a latent factor score derived from 2 memory items including rating of memory problem and concern about memory (mean [SD], 0[1]) for which ordinal thresholds were estimated.
Scale from 1 (never) to 5 (very often).
Scale from 1 (none) to 4 (major).
Scale from 1 (none) to 4 (very concerned).
Subjective Memory Concern
At a mean (SD) age of 37.75 (2.51) years (hereafter referred to as age 38 years), participants completed the Survey of Health study, which had a single subjective memory concern question25 that was converted into a continuous threshold liability score and standardized. At ages 56, 62, and 67 years, subjective memory concern was scored using 2 items combined into a factor score, fixing the loadings at 0.50 with standardized mean and variance of 0 and 1, respectively. Subjective memory concern was centered at age 38 years. Subjective memory concern questions and response options are shown in eTable 2 in Supplement 1.
Objective Memory
Objective memory was not assessed at age 38 years. At ages 56, 62, and 67 years, it was measured using a previously derived memory factor score based on 7 measures from a confirmatory twin-based factor analysis (California Verbal Learning Test-II) and immediate and delayed recall on Weschler Memory Scale-III Logical Memory and Visual Reproduction subtests.26 At follow-up assessments, scores were adjusted for practice effects as previously described.27 Amnestic mild cognitive impairment was defined using the Jak-Bondi approach.28,29 Memory impairment was defined as performing more than 1.5 SD below demographic-adjusted means on at least 2 tasks within the episodic memory domain. Prior to calculating those cutoffs, scores were adjusted for general cognitive ability at a mean age of 20 years to ensure that they reflected declines in performance rather than longstanding low ability.28,30 See the eMethods in Supplement 1 for further details.
General Cognitive Ability
Young adult general cognitive ability was measured with the Armed Forces Qualification Test administered at a mean age of 20 years. This test is correlated at approximately 0.85 with Wechsler IQ.31,32 See the eMethods in Supplement 1 for further details.
Affect-Related Measures
Depressive symptoms were measured using the 20-Item Center for Epidemiological Studies-Depression Scale.33 Anxiety was assessed with the Multidimensional Personality Questionnaire34 stress reaction scale at age 56 years and the 20-item trait form of the State-Trait Anxiety Inventory35,36 at ages 62 and 67 years. We also examined Multidimensional Personality Questionnaire negative emotionality, a validated measure of trait neuroticism34 assessed at age 56 years, and neuroticism and depression PRSs. PRSs were created using a standard clumping and thresholding approach in PLINK version 1.937,38 based on genome-wide association study summary statistics of neuroticism39 and depressive symptoms.40 See the eMethods in Supplement 1 for further details.
Alzheimer-Related Measures
We examined APOE genotype (ε4+ vs ε4−), AD PRS,39,41,42 and parental history of dementia. APOE is the major risk gene for late-onset AD43 but additional variance might be accounted for by the AD PRS. The same clumping and thresholding approach was used to create the AD PRS based genome-wide association study data (eMethods in Supplement 1).41,44 For parental history, participants were asked, “Did your biological mother or father ever have a serious memory difficulty referred to as senility, Alzheimer’s disease, or other kind of dementia?” Twin pairs with discordant reporting of parental history were coded as missing to avoid ambiguity.
Physical Health Conditions
Number of physical health conditions was based on the Charlson Index.45 Conditions included history of hypertension, heart attack, heart failure, stroke, peripheral vascular disease, thrombolysis, angina, diabetes, bronchitis, asthma, cancer, osteoarthritis, rheumatoid arthritis, and cirrhosis.
Statistical Analysis
Significant associations were determined by P < .05, also indicated by a 95% CI not including 0. Results presented include cases of mild cognitive impairment, but all results held up when individuals with mild cognitive impairment at VETSA baseline (age 56 years) were excluded.
Phenotypic Analyses
We used linear mixed models with random intercepts to characterize changes in subjective memory concern. Subjective memory concern was regressed onto baseline age and follow-up ages as our longitudinal time variable. Next, we tested multivariate associations using a structural equation model in Mplus version 8.4 (Muthén & Muthén). The model included autoregressive (unidirectional) paths from observations within the same measure over time and bidirectional paths that were set at ages 56, 62, and 67 years between subjective memory concern and objective memory, subjective memory concern and depressive symptoms, and subjective memory concern and anxiety and lagged paths from all variables to all variables at subsequent waves. The model was adjusted for age, race and ethnicity, number of health conditions at each wave, and young adult general cognitive ability. In the linear mixed model and structural equation model, analyses were adjusted for nonindependence of data due to twin relatedness. Race and ethnicity were self-reported based on categories provided by the investigators and were considered for this study to adjust for possible sociocultural differences in rating subjective memory concern.
When examining phenotypic correlations of subjective memory concern with genetic or family risk indicators (neuroticism PRS, depression PRS, AD PRS, APOE genotype, and parental history of dementia), we used the OpenMx version 2.9.9.1 software package in R version 3.4.1 (R Foundation)46,47 to estimate threshold liabilities for ordinal and binary variables. These polychoric correlations were also adjusted for twin relatedness and zygosity. As described previously,48 PRS analyses were restricted to individuals of European ancestry. Before analyses, PRSs were preadjusted for the first 3 principal components of ancestry to account for cryptic population structure (eMethods in Supplement 1). Results held up when adjusted for 20 principal components (not shown), but those may be subject to overfitting.
Biometric Twin Analyses
OpenMx version 2.9.9.1 was also used to fit twin models. The univariate ACE model decomposes the variance into variance explained by additive genetic influences (A), common environmental influences (C; environmental influences making paired twins similar), and unique environmental influences (E; environmental influences making paired twins different). This decomposition exploits the expected genetic and environmental correlations between monozygotic and dizygotic twin pairs. Monozygotic pairs are genetically identical whereas dizygotic pairs share, on average, half their genes. Shared environmental effects (C) are assumed equal in monozygotic and dizygotic twin pairs; and unique environmental effects (E) are, by definition, uncorrelated and include measurement error. Further description of univariate models is in the eMethods and eFigure 1 in Supplement 1.
We extended the univariate model to longitudinal multivariate models. As a reference model, we fit a multivariate ACE correlated factors model (Figure 1A), which reproduces all mean and variance-covariance information for the subjective memory concern variables while making no theoretical prediction regarding how genes and environments change over time. The independent pathways model (Figure 1B) predicts that genetic and environmental risk factors have separate pathways to the subjective memory concern scores. The common pathway model (Figure 1C) predicts that the covariance between all subjective memory concern scores is explained by a common liability while also allowing for some influences unique to each subjective memory concern score. The autoregressive model (Figure 1D) predicts that time-specific genetic or environmental influences accumulate and persist over time.49,50,51 Since subjective memory concern at age 38 years was not identical to the 3 later subjective memory concern measures, we allowed the first regression coefficient from age 38 years to age 56 years to vary freely. We also did not constrain the residual variance at age 38 years to equal the residual variances at the later time points.
Figure 1. Models Accounting for the Sources of Variance and Covariance in Subjective Memory Concern (SMC) Over Time.
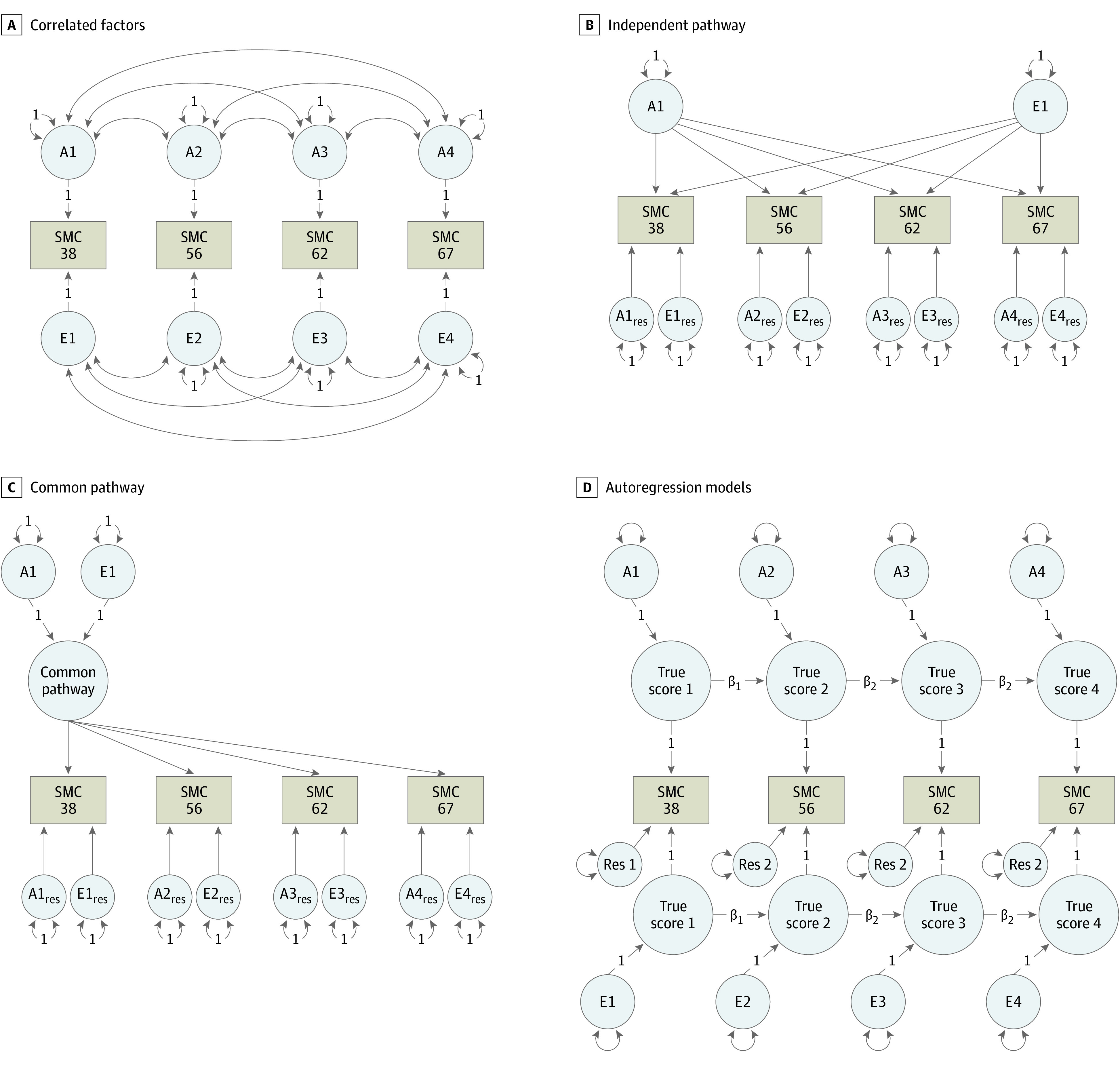
For brevity, only additive genetic (A) and unique environmental (E) influences are depicted at different time points (1, 2, 3, and 4, corresponding to ages 38, 56, 62, and 67 years, respectively). The correlated factors model is the reference model for comparison. In the autoregression model, the 2 regression coefficients starting at ages 56 and 62 years are constrained to be equal. To identify the autoregression model, the residual variances at ages 56, 62, and 67 years were constrained to be equal, while the factor loadings from the latent true scores to the observed scores were fixed to 1. Given the differences in assessment between SMC at ages 38, 56, 62, and 67 years, the first regression coefficient from age 38 to 56 years was allowed to vary freely. Likewise, the residual variance for SMC at age 38 years was allowed to vary freely. Res indicates residual variance component.
Model comparisons involved log-likelihood ratio tests to determine change in fit and the Akaike information criterion, which indicates optimal balance between goodness of fit and parsimony.52 Lower Akaike information criterion values indicate better model fit.
Results
Phenotypic Analyses
The sample included 1555 male adults examined at age 38 years, 520 at age 56 years (due to late introduction of subjective memory concern questions), 1199 at age 62 years, and 1192 at age 67 years. Race and ethnicity were reported as follows. American Indian or Alaska Native: 4 participants at age 38 years, 2 at age 56 years, 2 at age 62 years, and 5 at age 67 years; Asian: 0 participants at all time points; Black: 62 participants at age 38 years, 24 at age 56 years, 55 at age 62 years, and 72 at age 67 years; Native Hawaiian or Other Pacific Islander: 2 participants at age 38 years, 2 at age 56 years, 0 at age 62 years, and 9 at age 67 years; White: 1046 participants at age 38 years, 326 at age 56 years, 880 at age 62 years, and 1064 at age 67 years; more than 1 race: 18 participants at age 38 years, 6 at age 56 years, 18 at age 62 years, and 16 at age 67 years; Hispanic or Latino: 30 participants at age 38 years, 3 at age 56 years. 24 at age 62 years, and 31 at age 67 years; and not Hispanic or Latino: 1513 participants at age 38 years, 511 at age 56 years, 1170 at age 62 years, and 1161 at age 67 years.
Subjective memory concern was mostly stable and not associated with baseline age or time-varying age (eFigure 2 in Supplement 1). Phenotypic correlations of subjective memory concern with objective memory, depressive symptoms, anxiety, and negative emotionality are provided in eTables 3, 4, and 5 in Supplement 1. Phenotypic correlations between subjective memory concern at different ages are shown in eTable 6 in Supplement 1. Here we focus on the structural equation model results.
At age 56 years, higher subjective memory concern was concurrently associated with lower objective memory (β, −0.06; 95% CI, −0.09 to −0.03), greater depressive symptoms (β, 0.19; 95% CI, 0.16 to 0.23), and greater anxiety (β, 0.44; 95% CI, 0.35 to 0.52). Similar results were shown at ages 62 and 67 years, as shown in Figure 2. Subjective memory concern at age 38 years was associated with subjective memory concern at age 56 years (β, 0.17; 95% CI, 0.08 to 0.26). From ages 56 to 67 years, there were large, significant autoregressive paths across subjective memory concern (β, 0.62 to 0.70), objective memory (β, 0.62 to 0.69), depressive symptoms (β, 0.52), and anxiety (β, 0.31 to 0.79). At ages 56, 62, and 67 years, higher subjective memory concern was associated with lower concurrent objective memory (β, −0.10 to −0.06) and greater concurrent depressive symptoms (β, 0.19 to 0.30) and anxiety (β, 0.27 to 0.44). Subjective memory concern at any age did not predict later changes in objective memory, depressive symptoms, or anxiety, and none of these measures predicted later subjective memory concern. Subjective memory concern at age 38 years did predict greater depressive symptoms (β, 0.21; 95% CI, 0.16 to 0.27) and anxiety at age 56 years (β, 0.25; 95% CI, 0.19 to 0.30). Regarding covariates, older age was associated with worse objective memory at ages 56 years (β, −0.14; 95% CI, −0.19 to −0.09), 62 years (β, −0.07; 95% CI,−0.11 to −0.04), and 67 years (β, −0.06; 95% CI, −0.09 to− 0.03); more physical health conditions were associated with greater subjective memory concern at age 56 years (β, 0.14; 95% CI, 0.06 to 0.24) and 62 years (β, 0.11; 95% CI, 0.03 to 0.14). Race and ethnicity were not associated with any measures.
Figure 2. Structural Equation Model of Phenotypic Associations Among Subjective Memory Concern (SMC), Objective Memory, and Depressive Symptoms.
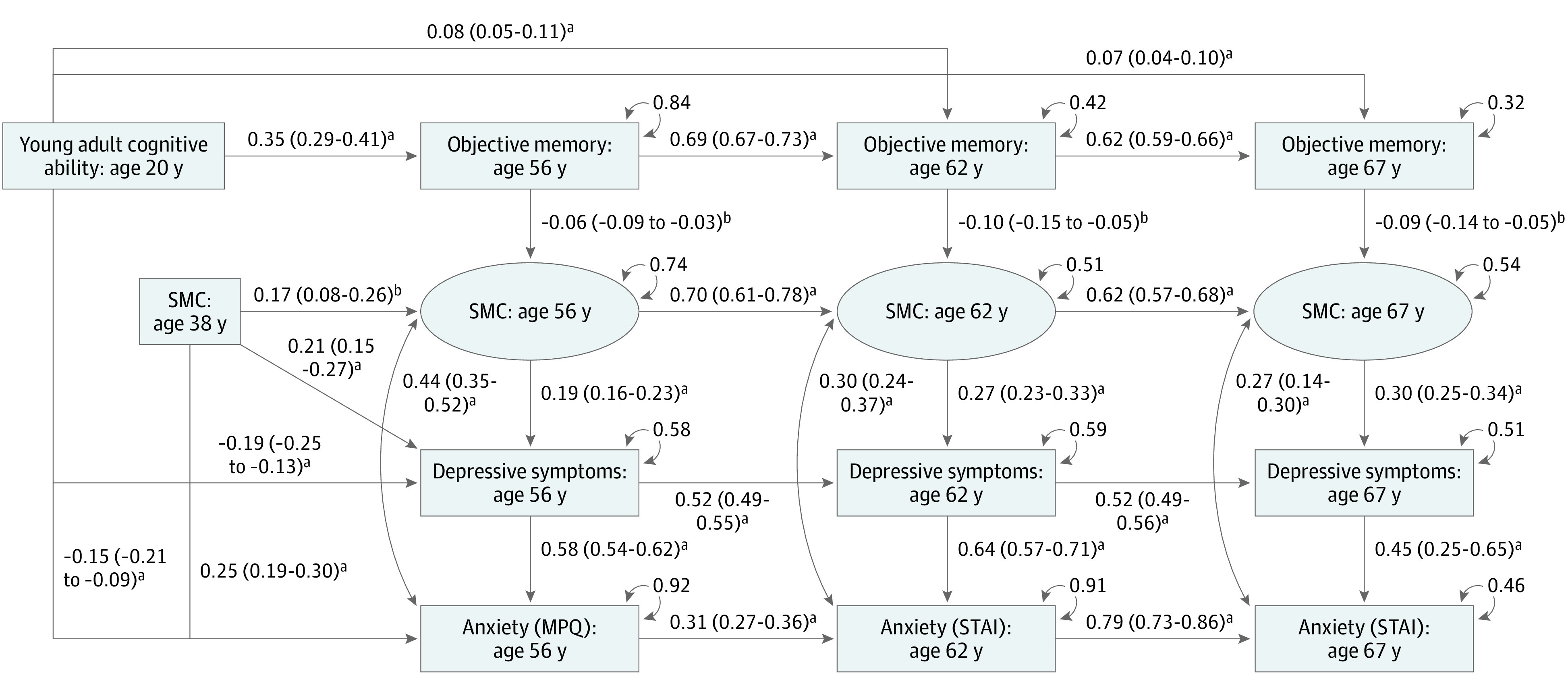
This structural equation model was additionally adjusted for age, race and ethnicity, physical health conditions, and clustering of twin pairs. For readability, only significant paths (P < .05) are shown. The model tested all possible bidirectional and lagged paths between variables. The labeled ages stand for the mean age of the sample at the wave when the variable was collected. Values are βs and 95% CIs. MPQ indicates Multidimensional Personality Questionnaire; STAI, State-Trait Anxiety Inventory.
aP < .001.
bP < .01.
As shown in eTable 7 in Supplement 1, the neuroticism PRS was correlated with subjective memory concern at ages 38 years (r, 0.10; 95% CI, 0.03 to 0.18) and 67 years (r, 0.09; 95% CI, 0.01 to 0.16). Subjective memory concern was not associated with the depression PRS or any AD genetic risk indicators.
Biometric Twin Analyses
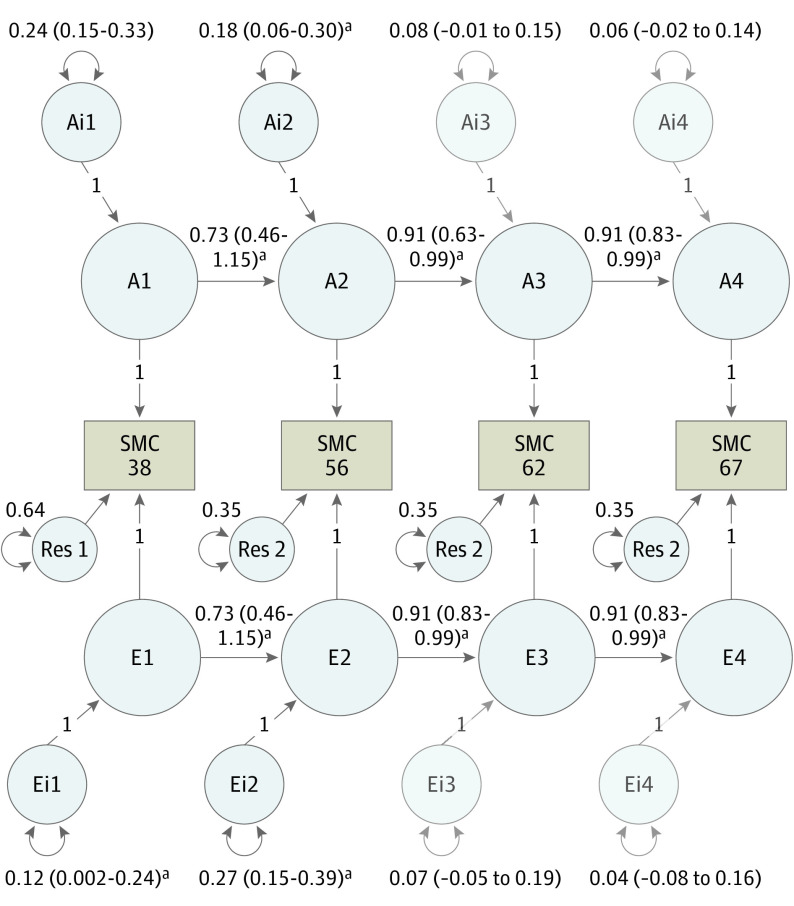
Univariate results are shown in eTable 8 in Supplement 1. Here we focus on the more powerful multivariate biometric analyses. An AE autoregressive model—with shared environmental influences dropped—provided the best fit (Table 2). As shown in Figure 3, the additive genetic and unique environmental factors associated with subjective memory concern at ages 38 and 56 years were large, enduring, and were associated with genetic and environmental variances at ages 62 and 67 years. Heritabilities (ie, additive genetic influences) for subjective memory concern are shown in eTable 9 in Supplement 1 and ranged from 0.26 to 0.34. There were significant new genetic and unique environmental associated factors at age 56 years but not at ages 62 and 67 years.
Table 2. Multivariate Model Fitting.
| Models | Estimated parameters, No. | −2LL | df | AIC | Δ−2LL | Δdf | P value |
|---|---|---|---|---|---|---|---|
| ACE-correlated factors (reference model)a | 34 | 11 788.83 | 4414 | 11 856.83 | NA | NA | NA |
| Independent pathways | 28 | 11 798.98 | 4420 | 11 854.98 | 1.15 | 6 | .12 |
| 1-Factor common pathway | 23 | 11 801.71 | 4426 | 11 847.71 | 12.89 | 12 | .38 |
| Autoregressive (reference model for submodels)a | 20 | 1804.89 | 4428 | 11 844.89 | 16.07 | 14 | .31 |
| AEa | 15 | 11 807.06 | 4433 | 11 837.06 | 2.16 | 5 | .83 |
| CE | 16 | 11 822.89 | 4432 | 11 854.89 | 17.99 | 4 | <.001 |
| E | 12 | 11 909.14 | 4436 | 11 933.14 | 104.25 | 8 | <.001 |
Abbreviations: −2LL, −2 × log-likelihood; ACE, additive genetic influences (A), common environmental influences (C), and unique environmental influences (E) environmental influences making paired twins different; AIC, Akaike information criteria; Δ, change; NA, not applicable.
Best-fitting model.
Figure 3. Best-Fitting Autoregressive Biometric Twin Model of Subjective Memory Concern (SMC).
A1-A4 estimate the cumulative additive genetic influences (A) at each time point (1, 2, 3, and 4, corresponding to ages 38, 56, 62, and 67, respectively); E1-E4 estimate the cumulative unique environmental influences (E) at each time point (eTable 9 in Supplement 1); Ai1 estimates the innovative (i) additive influences at age 38 years, while Ai2, Ai3, and Ai4 represent the magnitude of new genetic information at ages 56, 62, and 67 years, respectively; Ei1 estimates the magnitude of unique environmental influences at age 38 years; Ei2, Ei3, and Ei4 represent unique environmental influences at ages 56, 62, and 67 years, respectively; Res 1 and Res 2 represent residuals or unexplained variance measured at ages 62 and 67 years, respectively. Estimates on the directional paths between A1 to A4 and E1 to E4 represent βs for autoregressive associations. Residuals and βs for ages 56, 62, and 67 years were set to be equal without significant loss in model fit (P > .05).
aStatistical significance at P < .05.
Genetic correlations were obtained via the AE correlated factors model. There were significant genetic correlations for subjective memory concern across ages (r, 0.49 to 0.90) (eTable 10 in Supplement 1). At ages 56, 62, and 67 years, there were significant inverse genetic correlations between subjective memory concern and objective memory (r, −0.31 to −0.08) (eTable 3 in Supplement 1) and significant positive genetic correlations with depressive symptoms (r, 0.14 to 0.63) (eTable 4 in Supplement 1) and anxiety (r, 0.30 to 0.73) (eTable 5 in Supplement 1). Unique environmental correlations with subjective memory concern were also significant for objective memory (r, −0.20 to −0.06) (eTable 3 in Supplement 1), depressive symptoms (r, 0.12 to 0.39) (eTable 4 in Supplement 1), and anxiety (r, 0.30 to 0.70) (eTable 5 in Supplement 1). As shown in eTable 5 in Supplement 1, subjective memory concern was significantly phenotypically associated with negative emotionality (r, 0.26 to 0.34) with significant genetic (r, 0.51 to 0.63) and unique environmental correlations (r, 0.08 to 0.20).
Discussion
We examined heritability of subjective memory concern over 30 years and its association with objective memory performance, affect-related measures, and AD-related measures in community-dwelling men. Consistent with prior studies,12,13 subjective memory concern was moderately heritable at all ages examined. At any age, nearly one-third of the variance was accounted for by genetic influences. Our 30-year longitudinal data showed that genetic influences were best modeled as an autoregressive phenomenon wherein subjective memory concern was relatively stable, and subjective memory concern later in life (ie, ages 62 and 67 years) was largely due to genetic influences observable earlier in life (ie, ages 38 and 56 years). Phenotypically, subjective memory concern was significantly correlated with almost all affect-related measures but not with AD-related measures. Subjective memory concern was more strongly correlated both phenotypically and genetically with depressive symptoms and anxiety than with objective memory. There was also substantial variation in subjective memory concern according to unique environmental influences. A variety of lifestyle or other life experience variables may contribute to both genetic and unique environmental influences.
Our phenotypic findings showing stronger associations of subjective memory concern with affect-related measures than with objective memory are comparable to the findings of previous literature.3,10,15,16,17 There are genetic associations with depression and anxiety,53,54 but it is depressive symptoms that have been primarily examined in the literature. Here we found that both depressive symptoms and anxiety were associated with subjective memory concern, but the phenotypic and genetic associations were stronger for anxiety based on nonoverlapping confidence intervals of correlations (eTables 4 and 5 in Supplement 1). Moreover, the significant association of subjective memory concern with the neuroticism PRS but not the depression PRS may implicate the genetic influence of tendencies toward worry over low mood. Genes involved in neuroticism have been implicated in the development of brain structures for attention and learning, which may be involved in attentional biases toward worrying and distress,55,56,57,58 and subjective memory concern is also associated with worse structural integrity and functional connectivity in AD-related brain regions.59,60,61
Our results showing no association between subjective memory concern and objective memory decline are consistent with some studies17,62,63 but not others.7,64,65 Traits associated with subjective memory concern may partially account for this inconsistency. The stability of subjective memory concern over time suggests that it is more likely to be associated with relatively stable genetic traits rather than with changes in other measures. This again highlights the importance of accounting for potential traitlike components when attempting to quantify changes in subjective memory concern, such as those related to the onset of ADRD dementia, in order to isolate any state-related indicators of imminent risk of cognitive performance decline.
Limitations
This study has limitations. Results in this all-male sample may not be generalizable to women. For example, women reporting subjective memory concern may be more likely to have memory decline than men.66 Our sample also primarily identified as non-Hispanic White, similar to previous literature on this topic,8 and may not generalize to more diverse populations. For example, there is some evidence of an association between subjective memory concern and memory decline in Hispanic adults much older than the present sample.67,68 In addition, the sample in this study came from a community-dwelling population, which may not generalize to clinical settings. On the other hand, subjective memory concern at ages 38 and 56 years is almost certainly well before most people would be coming to a memory clinic. Moreover, we can think of no reason why a longstanding traitlike component of subjective memory concern would not also be present in individuals at a memory clinic. The maximum age in our sample was 73 years. One might speculate that subjective memory concern would be a more reliable indicator of AD risk in adults older than 73 years. However, given the results of our 30-year longitudinal twin analysis, it seems rather unlikely that subjective memory concern would no longer be heritable as these individuals continue to age. That said, it may seem intuitive that environmental factors, such as new illnesses, injuries, or isolation, would lead to lower heritability in older adults, but that is not necessarily the case. For example, although reduced heritability of brain ventricular volume with increasing age due to accumulated environmental influences seems to be a logical prediction, the proportion of genetic influences actually increases with increasing age.69 Being at much older age will not obviate the need to tease out the traitlike component of subjective memory concern. Although we did find a significant association with the neuroticism PRS, tests of PRS associations may have not been fully powered to detect very small effect sizes (r < 0.08), which are common for PRSs.70 The PRSs we assessed were also not exhaustive. There are many PRSs for psychiatric symptoms and disorders, which may be important to explore in the future. We also note that the age measure we used (38 years) rated frequency, whereas the later measures rated severity of memory concern. Future studies may benefit from differentiating between these.
Conclusions
Many AD research programs categorize cognitively unimpaired adults with subjective cognitive (most often memory) decline or concern as a specific subgroup because they are believed to be at elevated risk for near-term progression to mild cognitive impairment or dementia. This diagnostic label is only meaningful to the extent that subjective concern represents a statelike phenomenon related to AD. Our findings of a heritable traitlike component to subjective memory concern dating back to age 38 years are at odds with this conceptualization and suggest the need to account for longstanding perceptions of subjective memory if the goal is to use subjective memory concern to detect those at risk for impending cognitive decline or dementia.
eMethods
eTable 1. Descriptives of non-SMC study variables in the VETSA analytical sample across waves
eTable 2. Measures of subjective memory concern
eTable 3. Associations of subjective memory concern with objective memory
eTable 4. Associations of subjective memory concern with depressive symptoms
eTable 5. Associations of subjective memory concern with anxiety and negative emotionality
eTable 6. Phenotypic correlations between SMC at age 38, 56, 62, and 67
eTable 7. Associations of subjective memory concern with genetic risk indicators for neuroticism, depression, and Alzheimer’s disease
eTable 8. Subjective memory concern univariate model fitting results and comparisons
eTable 9. Variance components from best-fitting autoregressive biometric model describing genetic and unique environmental influences for SMC at each age
eTable 10. Genetic correlations (below diagonal) and unique environmental correlations (above diagonal) of SMC between ages 38, 56, 62, and 67
eFigure 1. Univariate variance decomposition of relative contribution of genetic and environmental influences on subjective memory concern
eFigure 2. Spaghetti plot showing the change in subjective memory concern (SMC) across age
Data sharing statement
References
- 1.Jessen F, Amariglio RE, van Boxtel M, et al. ; Subjective Cognitive Decline Initiative (SCD-I) Working Group . A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844-852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill NL, McDermott C, Mogle J, et al. Subjective cognitive impairment and quality of life: a systematic review. Int Psychogeriatr. 2017;29(12):1965-1977. doi: 10.1017/S1041610217001636 [DOI] [PubMed] [Google Scholar]
- 3.Hill NL, Mogle J, Wion R, et al. Subjective cognitive impairment and affective symptoms: a systematic review. Gerontologist. 2016;56(6):e109-e127. doi: 10.1093/geront/gnw091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fondell E, Townsend MK, Unger LD, et al. Physical activity across adulthood and subjective cognitive function in older men. Eur J Epidemiol. 2018;33(1):79-87. doi: 10.1007/s10654-017-0331-2 [DOI] [PubMed] [Google Scholar]
- 5.Griep Y, Hanson LM, Vantilborgh T, Janssens L, Jones SK, Hyde M. Can volunteering in later life reduce the risk of dementia? a 5-year longitudinal study among volunteering and non-volunteering retired seniors. PLoS One. 2017;12(3):e0173885. doi: 10.1371/journal.pone.0173885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirooka H, Nishiguchi S, Fukutani N, Tashiro Y, Nozaki Y, Aoyama T. Subjective cognitive decline and fall risk in community-dwelling older adults with or without objective cognitive decline. Aging Clin Exp Res. 2018;30(5):457-462. doi: 10.1007/s40520-017-0799-3 [DOI] [PubMed] [Google Scholar]
- 7.Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46(1):121-125. doi: 10.1212/WNL.46.1.121 [DOI] [PubMed] [Google Scholar]
- 8.Crumley JJ, Stetler CA, Horhota M. Examining the relationship between subjective and objective memory performance in older adults: a meta-analysis. Psychol Aging. 2014;29(2):250-263. doi: 10.1037/a0035908 [DOI] [PubMed] [Google Scholar]
- 9.Hill NL, Bhargava S, Bratlee-Whitaker E, Turner JR, Brown MJ, Mogle J. Longitudinal relationships between subjective cognitive decline and objective memory: depressive symptoms mediate between-person associations. J Alzheimers Dis. 2021;83(4):1623-1636. doi: 10.3233/JAD-210230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mogle J, Hill NL, Bhargava S, Bell TR, Bhang I. Memory complaints and depressive symptoms over time: a construct-level replication analysis. BMC Geriatr. 2020;20(1):57. doi: 10.1186/s12877-020-1451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson B, Bjork MP, Thorvaldsson VI. Rate my memory quite similar at age 40 and at age 70: findings in a swedish longitudinal study on subjective memory over a 30-year period. GeroPsych (Bern). 2020;33(4):235-244. doi: 10.1024/1662-9647/a000239 [DOI] [Google Scholar]
- 12.Singer JJ, MacGregor AJ, Cherkas LF, Spector TD. Where did I leave my keys? a twin study of self-reported memory ratings using the multifactorial memory questionnaire. Twin Res Hum Genet. 2005;8(2):108-112. doi: 10.1375/twin.8.2.108 [DOI] [PubMed] [Google Scholar]
- 13.Catts V, Thalamuthu A, Lee T, Crawford J, Sachdev P. Heritability of subjective cognitive decline in older Australian twins. Paper presented at: Behavior Genetics 2019. [Google Scholar]
- 14.Caracciolo B, Gatz M, Xu W, Pedersen NL, Fratiglioni L. Differential distribution of subjective and objective cognitive impairment in the population: a nation-wide twin-study. J Alzheimers Dis. 2012;29(2):393-403. doi: 10.3233/JAD-2011-111904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill NL, Mogle J, Bhargava S, et al. Longitudinal relationships among depressive symptoms and three types of memory self-report in cognitively intact older adults. Int Psychogeriatr. 2020;32(6):719-732. doi: 10.1017/S104161021900084X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill NL, Mogle J, Bhargava S, et al. Differences in the associations between memory complaints and depressive symptoms among black and white older adults. J Gerontol B Psychol Sci Soc Sci. 2020;75(4):783-791. doi: 10.1093/geronb/gby091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustavson DE, Jak AJ, Elman JA, et al. How well does subjective cognitive decline correspond to objectively measured cognitive decline? assessment of 10–12 year change. J Alzheimers Dis. 2021;83(1):291-304. doi: 10.3233/JAD-210123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremen WS, Fennema-Notestine C, Eyler LT, et al. Genetics of brain structure: contributions from the Vietnam Era Twin Study of Aging. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(7):751-761. doi: 10.1002/ajmg.b.32162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremen WS, Franz CE, Lyons MJ. Current status of the Vietnam Era Twin Study of Aging (VETSA). Twin Res Hum Genet. 2019;22(6):783-787. doi: 10.1017/thg.2019.125 [DOI] [PubMed] [Google Scholar]
- 20.Kremen WS, Thompson-Brenner H, Leung YM, et al. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA). Twin Res Hum Genet. 2006;9(6):1009-1022. doi: 10.1375/twin.9.6.1009 [DOI] [PubMed] [Google Scholar]
- 21.Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: what we have learned. Harv Rev Psychiatry. 2001;9(6):267-279. [PubMed] [Google Scholar]
- 22.Schoeneborn CA, Heyman KM. Health characteristics of adults aged 55 years and over: United States, 2004-2007. National Health Statistics Reports . Published July 8, 2009. Accessed August 29, 2023. http://caregiverslibrary.org/Portals/0/National_Health_Statistics_Aug_3_2009_nhsr016.pdf [PubMed]
- 23.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 24.Westland JC. Lower bounds on sample size in structural equation modeling. Electron Commerce Res Appl. 2010;9(6):476-487. doi: 10.1016/j.elerap.2010.07.003 [DOI] [Google Scholar]
- 25.Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105(4):368-373. [PMC free article] [PubMed] [Google Scholar]
- 26.Kremen WS, Panizzon MS, Franz CE, et al. Genetic complexity of episodic memory: a twin approach to studies of aging. Psychol Aging. 2014;29(2):404-417. doi: 10.1037/a0035962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elman JA, Jak AJ, Panizzon MS, et al. Underdiagnosis of mild cognitive impairment: a consequence of ignoring practice effects. Alzheimers Dement (Amst). 2018;10:372-381. doi: 10.1016/j.dadm.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368-375. doi: 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jak AJ, Preis SR, Beiser AS, et al. Neuropsychological criteria for mild cognitive impairment and dementia risk in the Framingham Heart Study. J Int Neuropsychol Soc. 2016;22(9):937-943. doi: 10.1017/S1355617716000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granholm EL, Panizzon MS, Elman JA, et al. Pupillary responses as a biomarker of early risk for Alzheimer’s disease. J Alzheimers Dis. 2017;56(4):1419-1428. doi: 10.3233/JAD-161078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons MJ, Panizzon MS, Liu W, et al. A longitudinal twin study of general cognitive ability over four decades. Dev Psychol. 2017;53(6):1170-1177. doi: 10.1037/dev0000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons MJ, York TP, Franz CE, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol Sci. 2009;20(9):1146-1152. doi: 10.1111/j.1467-9280.2009.02425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 2016;1(3):385-401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 34.Tellegen A, Waller NG. Exploring personality through test construction: development of the Multidimensional Personality Questionnaire. In Boyle GJ, Matthews G, Saklofske DH, eds. Personality Measurement and Testing. The SAGE Handbook of Personality Theory and Assessment; vol 2. Sage Publications; 2008:261-292. doi: 10.4135/9781849200479.n13 [DOI] [Google Scholar]
- 35.Spielberger CD. The State/Trait Anxiety Inventory for Adults. Mind Garden; 2005. [Google Scholar]
- 36.Spielberger CD. Manual for the state-trait anxiety inventory (STAI) (form Y). Consulting Psychologists Press; 1983. [Google Scholar]
- 37.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4(1):7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen NL, Gatz M, Finch BK, et al. IGEMS: the consortium on interplay of genes and environment across multiple studies—an update. Twin Res Hum Genet. 2019;22(6):809-816. doi: 10.1017/thg.2019.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luciano M, Hagenaars SP, Davies G, et al. Association analysis in over 329,000 individuals identifies 116 independent variants influencing neuroticism. Nat Genet. 2018;50(1):6-11. doi: 10.1038/s41588-017-0013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okbay A, Baselmans BM, De Neve J-E, et al. ; LifeLines Cohort Study . Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet. 2016;48(6):624-633. doi: 10.1038/ng.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloyd-Jones LR, Zeng J, Sidorenko J, et al. Improved polygenic prediction by bayesian multiple regression on summary statistics. Nat Commun. 2019;10(1):5086. doi: 10.1038/s41467-019-12653-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. ; European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452-1458. doi: 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921-923. doi: 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- 44.Kunkle BW, Grenier-Boley B, Sims R, et al. ; Alzheimer Disease Genetics Consortium (ADGC); European Alzheimer’s Disease Initiative (EADI); Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE); Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES) . Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414-430. doi: 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 46.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers; 1992. doi: 10.1007/978-94-015-8018-2 [DOI] [Google Scholar]
- 47.Boker S, Neale M, Maes H, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306-317. doi: 10.1007/s11336-010-9200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logue MW, Panizzon MS, Elman JA, et al. Use of an Alzheimer’s disease polygenic risk score to identify mild cognitive impairment in adults in their 50s. Mol Psychiatry. 2019;24(3):421-430. doi: 10.1038/s41380-018-0030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mcgue M, Bacon S, Lykken DT. Personality stability and change in early adulthood—a behavioral genetic-analysis. Dev Psychol. 1993;29(1):96-109. doi: 10.1037/0012-1649.29.1.96 [DOI] [Google Scholar]
- 50.Boomsma DI, Martin NG, Molenaar PC. Factor and simplex models for repeated measures: application to two psychomotor measures of alcohol sensitivity in twins. Behav Genet. 1989;19(1):79-96. doi: 10.1007/BF01065885 [DOI] [PubMed] [Google Scholar]
- 51.Boomsma DI, Molenaar PC. The genetic analysis of repeated measures. I. simplex models. Behav Genet. 1987;17(2):111-123. doi: 10.1007/BF01065991 [DOI] [PubMed] [Google Scholar]
- 52.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716-723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 53.Morneau-Vaillancourt G, Coleman JRI, Purves KL, et al. The genetic and environmental hierarchical structure of anxiety and depression in the UK Biobank. Depress Anxiety. 2020;37(6):512-520. doi: 10.1002/da.22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fergusson DM, Horwood LJ, Lawton JM. The relationships between neuroticism and depressive symptoms. Soc Psychiatry Psychiatr Epidemiol. 1989;24(6):275-281. doi: 10.1007/BF01788029 [DOI] [PubMed] [Google Scholar]
- 55.Opel N, Amare AT, Redlich R, et al. Cortical surface area alterations shaped by genetic load for neuroticism. Mol Psychiatry. 2020;25(12):3422-3431. doi: 10.1038/s41380-018-0236-9 [DOI] [PubMed] [Google Scholar]
- 56.Wright CI, Williams D, Feczko E, et al. Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex. 2006;16(12):1809-1819. doi: 10.1093/cercor/bhj118 [DOI] [PubMed] [Google Scholar]
- 57.Zufferey V, Donati A, Popp J, et al. Neuroticism, depression, and anxiety traits exacerbate the state of cognitive impairment and hippocampal vulnerability to Alzheimer’s disease. Alzheimers Dement (Amst). 2017;7:107-114. doi: 10.1016/j.dadm.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience. brain structure and the big five. Psychol Sci. 2010;21(6):820-828. doi: 10.1177/0956797610370159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27(12):1751-1756. doi: 10.1016/j.neurobiolaging.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 60.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834-842. doi: 10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hafkemeijer A, Altmann-Schneider I, Oleksik AM, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3(4):353-362. doi: 10.1089/brain.2013.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flicker C, Ferris SH, Reisberg B. A longitudinal study of cognitive function in elderly persons with subjective memory complaints. J Am Geriatr Soc. 1993;41(10):1029-1032. doi: 10.1111/j.1532-5415.1993.tb06448.x [DOI] [PubMed] [Google Scholar]
- 63.Hertzog C, Hülür G, Gerstorf D, Pearman AM. Is subjective memory change in old age based on accurate monitoring of age-related memory change? evidence from two longitudinal studies. Psychol Aging. 2018;33(2):273-287. doi: 10.1037/pag0000232 [DOI] [PubMed] [Google Scholar]
- 64.Wion R, Hill N, Bell T, Mogle J, Yates J, Bhang I. Does cognitive self-report measure type differentially predict cognitive decline? a systematic review. Innov Aging. 2020;4(suppl 1):292. doi: 10.1093/geroni/igaa057.936 [DOI] [Google Scholar]
- 65.Hülür G, Willis SL, Hertzog C, Schaie KW, Gerstorf D. Is subjective memory specific for memory performance or general across cognitive domains? findings from the Seattle Longitudinal Study. Psychol Aging. 2018;33(3):448-460. doi: 10.1037/pag0000243 [DOI] [PubMed] [Google Scholar]
- 66.Drouin SM, McFall GP, Dixon RA. In multiple facets of subjective memory decline sex moderates memory predictions. Alzheimers Dement (Amst). 2020;12(1):e12089. doi: 10.1002/dad2.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakhla MZ, Cohen L, Salmon DP, et al. Self-reported subjective cognitive decline is associated with global cognition in a community sample of Latinos/as/x living in the United States. J Clin Exp Neuropsychol. 2021;43(7):663-676. doi: 10.1080/13803395.2021.1989381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell TR, Pope CN, Downer B, Barba C, Crowe M. Pain associates with subjective memory problems and cognition in older Puerto Rican adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2022;29(6):985-999. doi: 10.1080/13825585.2021.1947957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kremen WS, Panizzon MS, Neale MC, et al. Heritability of brain ventricle volume: converging evidence from inconsistent results. Neurobiol Aging. 2012;33(1):1-8. doi: 10.1016/j.neurobiolaging.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bogdan R, Baranger DAA, Agrawal A. Polygenic risk scores in clinical psychology: Bridging genomic risk to individual differences. Annu Rev Clin Psychol. 2018;14:119-157. doi: 10.1146/annurev-clinpsy-050817-084847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Descriptives of non-SMC study variables in the VETSA analytical sample across waves
eTable 2. Measures of subjective memory concern
eTable 3. Associations of subjective memory concern with objective memory
eTable 4. Associations of subjective memory concern with depressive symptoms
eTable 5. Associations of subjective memory concern with anxiety and negative emotionality
eTable 6. Phenotypic correlations between SMC at age 38, 56, 62, and 67
eTable 7. Associations of subjective memory concern with genetic risk indicators for neuroticism, depression, and Alzheimer’s disease
eTable 8. Subjective memory concern univariate model fitting results and comparisons
eTable 9. Variance components from best-fitting autoregressive biometric model describing genetic and unique environmental influences for SMC at each age
eTable 10. Genetic correlations (below diagonal) and unique environmental correlations (above diagonal) of SMC between ages 38, 56, 62, and 67
eFigure 1. Univariate variance decomposition of relative contribution of genetic and environmental influences on subjective memory concern
eFigure 2. Spaghetti plot showing the change in subjective memory concern (SMC) across age
Data sharing statement