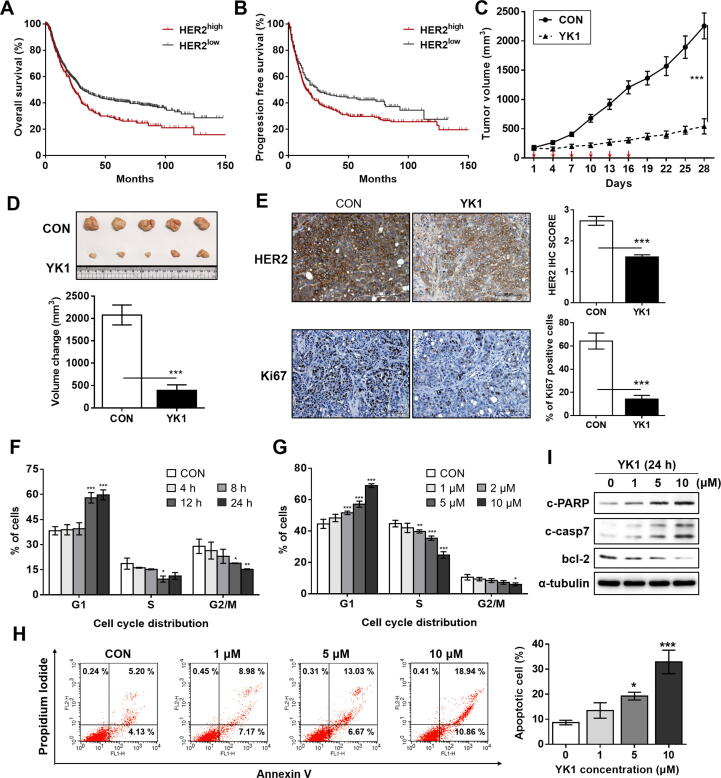
Fig. 4.
Clinical relevance of HER2 overexpression and promising in vitro and in vivo anticancer activities of YK1 based upon significant downregulation of HER2. (A, B) Overall survival (log rank test, P = 0.00088) (A) and progression-free survival (log rank test, P = 0.019) (B) of 1,065 gastric cancer patients were analyzed, and the Kaplan-Meier plots were generated by the Kaplan-Meier Plotter (http://www.kmplot.com). (C) Tumor regression was markedly promoted by YK1 in an NCI-N87-xenograft mouse model (n = 5 per group; intravenous (IV) injection of YK1 (4 mg/kg every 3 days). (D) Photograph of the tumors collected from the vehicle- and YK1–treated mice (upper panel). Tumor volumes were evaluated at the indicated time points by measuring the length and width of the tumor with callipers using the equation (length × width2) / 2 (mean ± S.E.M.) (lower panel). Student’s t-test, ***P < 0.001 vs CON. (E) IHC stains of HER2 and Ki67 (proliferation marker) in the tumors. IHC score quantification was conducted using Image J (10 independent fields per sample were assessed, mean ± S.D.). Student’s t test, ***P < 0.001 vs CON. (F, G) G1 arrest was induced by YK1 with time-dependency (10 μM at each time point; n = 3) (F) concentration-dependency (24 h treatment at the indicated concentrations, n = 3) (G). ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001 vs CON. (H) Proportions of apoptotic cell fractions were remarkably increased with treatment of YK1 in a dose-dependent manner (24 h treatment at indicated concentrations). (I) YK1–mediated induction of apoptosis was confirmed by increased pro-apoptotic (e.g., c-PARP and c-casp7) and decreased anti-apoptotic (e.g., bcl-2) markers (24 h treatment at indicated concentrations).