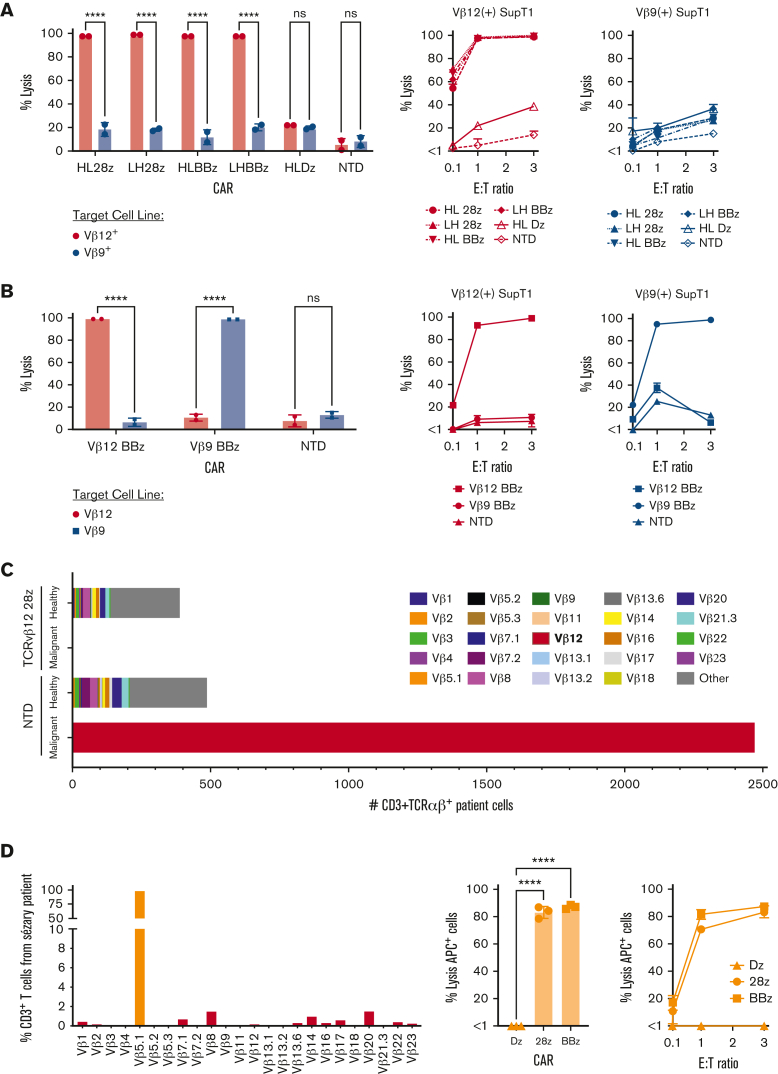
Figure 4.
TCRvβ-CARTs effectively and specifically lyse target cells in vitro. (A) Cytotoxicity against SupT1s engineered to express a TCR containing either TCRvβ12 or TCRvβ9 by TCRvβ12-CARTs. Lysis was determined using a bioluminescence-based assay at various E:T ratios. Representative plots from 1 donor at 1:1 E:T ratio (left) or E:T ratios ranging from 0.1:1 to 3:1 (right) (∗∗∗∗P < .0001 by 2-way ANOVA with Sídák multiple comparison test). (B) Killing of engineered SupT1s by either TCRvβ12-BBz or TCRvβ9-BBz CARTs. Representative plots from 1 donor at 3:1 E:T ratio (left) or E:T ratios ranging from 0.1:1 to 3:1 (right) (∗∗∗∗P < .0001 by 2-way ANOVA with Sídák multiple comparison test). (C) Quantification of the number of patient cells expressing TCRvβ families remaining after coculture with either NTD T cells or TCRvβ12-28z–CARTs as identified by flow cytometry. TCRvβ12-expressing cells (red) represent the malignant clone. (D) Lysis against a patient sample identified to have a TCRvβ5.1 dominant clone (left) by T cells engineered to express CAR constructs targeting TCRvβ5.1. Representative plots at 3:1 E:T ratio (middle) or various E:T ratios (right) (∗∗∗∗P < .0001 by one-way ANOVA with Dunnett multiple comparison test). Data for all figures are representative of experiments repeated 3 times with CARTs derived from different healthy donors.