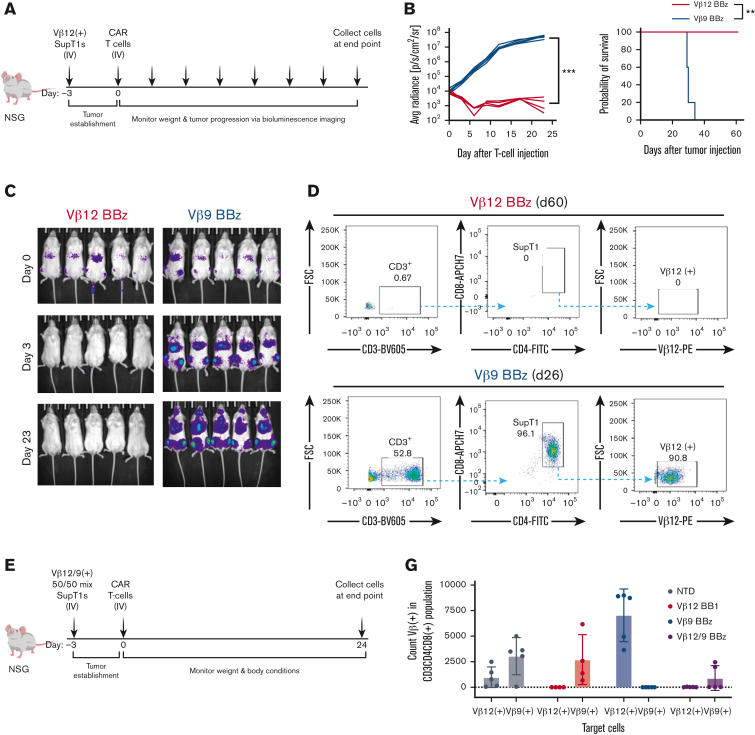
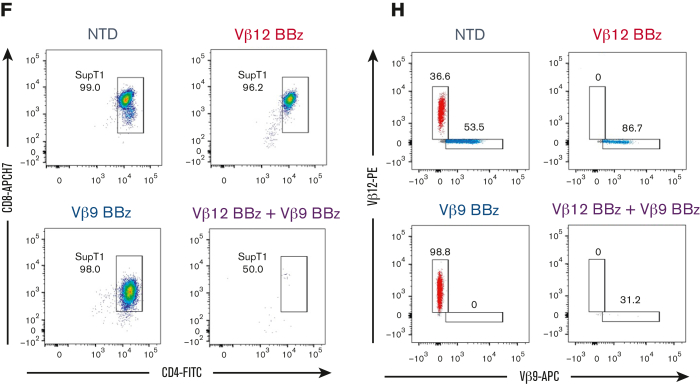
Figure 6.
TCRvβ-CARTs specifically reduce tumor burden in vivo. (A) Experimental setup of the in vivo experiment with a homogenous target population. (B) Quantification of luminescence and survival of mice (n = 5 per group) (∗∗∗P = .0008 by mixed-effects analysis, ∗∗P = .0039 by Gehan-Breslow-Wilcoxon test). (C) In vivo imaging system imaging of tumor cells after treatment with TCRvβ12-CARTs (left) or TCRvβ9-CARTs (right) over the course of the experiment. (D) Representative dot plots of CD3+CD4+CD8+TCRvβ12+ SupT1s identified in brain tissue collected at the respective end points. (E) Experimental setup of the in vivo experiment with a heterogeneous target population. (F) Representative dot plots of CD8+CD4+ SupT1s identified in brain tissue collected 24 days after T-cell infusion. Each plot represents an individual mouse. (G) Quantified TCRvβ12- and TCRvβ9-expressing cells within the identified SupT1s in all mice measured by flow cytometry. (H) Representative plots of TCRvβ12 and TCRvβ9 expression of the identified SupT1s (n = 5 per group). APC, activated protein C; FSC, forward scatter.