Abstract
Objectives
Subjective cognitive decline (SCD) and amnestic mild cognitive impairment (aMCI) are known as the preclinical and early stage of Alzheimer's disease (AD). The dorsal attention network (DAN) is mainly responsible for the “top‐down” attention process. However, previous studies mainly focused on single functional modality and limited structure. This study aimed to investigate the multimodal alterations of DAN in SCD and aMCI to assess their diagnostic value in preclinical and early‐stage AD.
Methods
Resting‐state functional magnetic resonance imaging (MRI) was carried out to measure the fractional amplitude of low‐frequency fluctuation (fALFF), regional homogeneity (ReHo), and functional connectivity (FC). Structural MRI was used to calculate the gray matter volume (GMV) and cortical thickness. Moreover, receiver‐operating characteristic (ROC) analysis was used to distinguish these alterations in SCD and aMCI.
Results
The SCD and aMCI groups showed both decreased ReHo in the right middle temporal gyrus (MTG) and decreased GMV compared to healthy controls (HCs). Especially in the SCD group, there were increased fALFF and increased ReHo in the left inferior occipital gyrus (IOG), decreased fALFF and increased FC in the left inferior parietal lobule (IPL), and reduced cortical thickness in the right inferior temporal gyrus (ITG). Furthermore, functional and structural alterations in the SCD and aMCI groups were closely related to episodic memory (EM), executive function (EF), and information processing speed (IPS). The combination of multiple indicators of DAN had a high accuracy in differentiating clinical stages.
Conclusions
Our current study demonstrated functional and structural alterations of DAN in SCD and aMCI, especially in the MTG, IPL, and SPL. Furthermore, cognitive performance was closely related to these significant alterations. Our study further suggested that the combined multiple indicators of DAN could be acted as the latent neuroimaging markers of preclinical and early‐stage AD for their high diagnostic value.
Keywords: amnestic mild cognitive impairment, dorsal attention network, resting‐state functional magnetic resonance imaging, structural magnetic resonance imaging, subjective cognitive decline
Our current study demonstrated obviously functional and structural alterations of DAN in SCD and aMCI. We found that the abnormalities of the functional alterations were especially located in the IPL, IOG, and MTG, whereas structural alterations were mainly in the SPL and ITG. Furthermore, cognitive performance was closely related to these significant alterations. Our study further suggested that the combined multiple indicators of DAN could be acted as the latent neuroimaging markers of preclinical and early‐stage AD for their high diagnostic value.
1. INTRODUCTION
Subjective cognitive decline (SCD) is known as the preclinical stage of Alzheimer's disease (AD). 1 SCD is used to represent the condition of the elderly who are considered to show subjectively declined in the absence of objective cognitive impairment. 2 Amnestic mild cognitive impairment (aMCI) is recognized as the early stage of AD. 3 AMCI is a syndrome with cognitive degeneration, especially impaired memory. 4 They are both high‐risk stages for conversion to AD. 5 With the lack of effective treatment for AD, identifying sensitive diagnostic markers in the preclinical and early‐stage AD is crucial. 6
Attention is a choice activity of consciousness, representing the initial link of cognitive processing. 7 There has been a lot of evidence that early dementia patients have the clinical manifestations of attention deficit. 8 , 9 , 10 As the most common neurodegenerative disease, the early clinical manifestation of AD is usually memory impairment. However, attention, as the initial stage of cognition, is often not easily perceived, which is also the starting point of this study. The resting‐state functional magnetic resonance imaging (rs‐fMRI) is an indispensable auxiliary diagnostic method to detect functional neuroimaging changes, 11 especially in the brain networks. 12 The dorsal attention network (DAN) has been associated with working and episodic memory and is mainly responsible for the “top‐down” attention process. 13 Moreover, the latest research shows that DAN is highly predictive on cognitive ability relative to other networks. 14
The fractional amplitudes of low‐frequency fluctuation (fALFF), regional homogeneity (ReHo), and functional connectivity (FC) are the three indicators measured by the rs‐fMRI. 15 They, respectively, indicate the spontaneous activity of neurons, 16 the consistency of neuronal activity, 17 and neurophysiological activities in two or more spatially corresponding regions. 18 Furthermore, structural magnetic resonance imaging (MRI) is also sensitive to examine structural brain alterations, including gray matter volume (GMV) and cortical thickness. 19 In this article, we chose the combination of functional and structural measurements, a more comprehensive approach, for a better understanding of the neuroimages of DAN in SCD and aMCI.
Previous research mainly focused on the FC interactions between DAN and other networks in the spectrum of AD. 20 , 21 The explorations of DAN were simple with single functional modality and limited structure. 22 Furthermore, previous studies lacked the changes of these indicators within DAN for the diagnosis and differentiation of SCD and aMCI. Taken over, these problems motivated us to extend previous research. We applied more functional indicators such as fALFF and ReHo and the structural indicators of GMV and cortical thickness. Additionally, we further explored the cognition associated with these alterations. We also employed the multimodal alterations of DAN to assess their diagnostic value in preclinical and early‐stage AD.
2. METHODS
2.1. Subjects
All subjects were from Nanjing Brain Hospital‐Alzheimer's Disease Spectrum Neuroimaging Project Version 2 (NBH‐ADsnp‐2), which has been approved by the responsible Human Participant Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University (2018‐KY010‐01, 2020‐KY010‐42). 23 , 24 All subjects in NBH‐ADsnp‐2, who were all Han Chinese and right‐handed, were recruited initially from hospitals and local communities by advertising and by means of broadcasting in the Appendix S1. All subjects participated voluntarily and obtained written informed consent. A total of 132 individuals from the baseline period of the NBH‐ADnsp‐2 database were initially enrolled. Among them, three aMCI participants, two SCD participants, and two HC participants were excluded due to excessive head motion (cumulative translation or rotation of >3.0 mm or 3.0°); 125 individuals were ultimately summarized in this study, including 40 aMCI, 43 SCD, and 42 healthy controls (HCs).
Inclusion criteria of SCD subjects were identified meeting the published SCD research criteria proposed by the Subjective Cognitive Decline Initiative (SCD‐I), 1 and the detailed inclusion criteria have been described as follows: (a) self‐reported persistent memory decline, which was confirmed by an informant; (b) Subjective Cognitive Decline Questionnaire (SCD‐Q) score > 5 25 , 26 , 27 ; (c) performance within the normal range on MMSE and MoCA (adjusted for age and education); (d) Clinical Dementia Rating (CDR) = 0; and (e) subjects aged between 50 and 80 years old.
Inclusion criteria of aMCI subjects were identified meeting the diagnostic criteria defined by Petersen et al. 28 as well as the revised consensus standards presented by Winblad et al., 29 and the detailed inclusion criteria have been described as follows: (a) memory complaint preferably corroborated by an informant or the subject for more than 3 months; (b) objective memory impairment adjusted for age and educational level; (c) normal general cognitive function of MMSE score equal or above 24; (d) no or minimal impairment in daily living activities; (e) CDR = 0.5; (f) subjects aged between 50 and 80 years old; and (g) absence of dementia symptoms that were not sufficient to meet the criteria of the National Institute of Neurological and Communicative Disorders and Stroke or the AD and Related Disorders Association criteria for AD.
The detailed exclusion criteria for all subjects had been described in the Appendix S2.
2.2. Demographics and neuropsychological assessments
We collected demographic information (age, gender, and education levels) of all subjects. All subjects approved neuropsychological assessments by professional neuropsychologists. General cognitive function was assessed by Mini‐Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Mattis Dementia Rating Scale‐2 (MDRS‐2). At the same time, the evaluation of four cognitive domains, including episodic memory (EM), executive function (EF), information processing speed (IPS), and visuospatial function (VF), was conducted based on neuropsychological assessments in Table 1. Division and assessment details were listed in the Appendix S3.
TABLE 1.
Demographics and neuropsychological characteristics of HCs, SCD, and aMCI three groups.
| HCs (n = 42) | SCD (n = 43) | aMCI (n = 40) | p | |
|---|---|---|---|---|
| Age (years) | 63.82 (7.35) | 63.63 (7.10) | 64.65 (7.99) | 0.673 a |
| Gender (M/F) | 16/26 | 10/33 | 18/22 | 0.104 b |
| Education level (years) | 12.36 (2.41) | 11.85 (3.26) | 11.89 (3.08) | 0.679 a |
| MMSE | 29.00 (28.00, 30.00) | 28.00 (27.00, 29.00) | 27.50 (26.00, 29.00) | 0.055 c |
| MoCA | 26.00 (25.00, 28.00) | 25.00 (24.00, 27.00) | 24.00 (22.00, 25.00) d ,*, e ,**, f ,*** | 0.000 c |
| MDRS‐2 | 142.00 (140.75, 144.00) | 141.00 (139.00, 143.00) | 138.00 (135.25, 141.00) d ,*, e ,**, f ,*** | 0.001 c |
| Composite Z scores of each cognitive domain | ||||
| Episodic memory | 0.28 (0.59) | 0.21 (0.62) | −0.52 (0.55) e ,***, f ,*** | 0.000 a |
| Executive function | 0.16 (0.53) | 0.27 (0.52) | −0.46 (0.56) e ,***, f ,*** | 0.000 a |
| Information processing speed | 0.12 (0.75) | 0.17 (0.70) | −0.31 (0.71) e ,**, f ,* | 0.006 a |
| Visuospatial function | 0.30 (−0.18, 0.64) | 0.15 (−0.37, 0.64) | 0.01 (−0.50, 0.64) | 0.141 c |
Note: Numbers are given as means (standard deviation, SD) or median (interquartile range, IQR).
Abbreviations: aMCI, amnestic mild cognitive impairment; HCs, healthy controls; MDRS‐2, Mattis Dementia Rating Scale‐2; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment test; SCD, subjective cognitive decline.
One‐way ANOVA.
Chi‐square test.
Kruskal‐Wallis H‐test.
Post hoc analyses showed a significant group difference between HCs and SCD.
Post hoc analyses showed a significant group difference between SCD and aMCI.
Post hoc analyses showed a significant group difference between HCs and aMCI.
*p < 0.05, **p < 0.01, ***p < 0.001.
2.3. Image acquisition
All MRI scans were performed on a 3.0T scanner. The detailed image acquisition parameters, including rs‐fMRI images and structural MRI images, were provided in the Appendix S4.
2.4. Rs‐fMRI data preprocessing
All rs‐fMRI data were preprocessed in the matlab2013b (http://www.mathworks.com/products/matlab/) and Data Processing Assistant for Resting‐State FMRI (DPARSF, http://restfmri.net/forum/DPARSF). 30 The first 10 time points were removed before slice time and head motion correction. Then, we detached subjects with excessive head motion (cumulative translation or rotation >3.0 mm or 3.0°). Subsequently, structural images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) partitions. We used the Diffeomorphic Anatomical Registration Through Exponentiated Lie (DARTEL) algorithm to normalize. 31 After normalization, they were regressed with the Friston 24‐parameter model and resampled 3 × 3 × 3 mm3 into Montreal Neurological Institute (MNI). Finally, the fMRI data were spatially smoothed and temporal band‐pass filtered. 32
2.5. Structural MRI data preprocessing
The structural T1 images were preprocessed in the Data Processing Analysis Brain Imaging (DPABI). 30 The structural images were segmented into GM, WM, and CSF. The resolution of the resulting GM images was 1.5 × 1.5 × 1.5 mm3. Then, spatially smoothed by a Gaussian kernel of 6 mm3 full‐width at half maximum (FWHM) to reduce spatial noise. The FreeSurfer software also analyzed the cortical thickness with T1 images. The standard processing procedures included were in the Appendix S5.
2.6. Definition of DAN mask
According to the previous meta‐analysis, the brain areas of DAN were summarized. 15 Then, we used the WFU_PickAtlas toolbox (www.ansir.wfubmc.edu) in the matlab2013b to make the DAN mask. The DAN mask included bilateral precentral gyrus (PreCG), superior frontal gyrus (SFG), middle frontal gyrus (MFG), superior parietal lobule (SPL), intraparietal sulcus (IPS), inferior occipital gyrus (IOG), inferior temporal gyrus (ITG), and MTG. 33 , 34 , 35 , 36 , 37 , 38 , 39
2.7. FALFF, ReHo, and FC measurement
The calculation of the fALFF value was performed in the DPABI software. The fALFF data were temporally band‐pass filtered (from 0.01 to 0.10 Hz) to reduce the low‐frequency drift and high‐frequency respiratory and cardiac noise. For each given voxel, the time series were converted to the frequency domain, and the power spectrum was obtained. The fALFF results were transformed using Fisher's z transformation fractional amplitude of low‐frequency fluctuation (zfALFF) and used for the following analysis. 40
The ReHo index was computed in a voxel‐wise manner using Kendall's correlation coefficient of a given voxel and its adjacent voxel (26 voxels) from the unsmoothed time series. Each ReHo map was also transformed using Fisher's z transformation regional homogeneity (zReHo). These zReHo maps were spatially smoothed with a 6‐mm Gaussian kernel FWHM to reduce the effect of noise. We earned smoothed zReHo (szReHo) and used it for subsequent analysis. 41
Seed‐based FC analysis was carried out to explore the alternations of DAN. According to a previous study, the right IPS was a vital region in the DAN. We made a 6‐mm spherical region of interest (ROI) centered in the right IPS (MNI space: 32, −56, 54). 42 , 43 The voxel‐wise Pearson correlation analysis was extracted between the average time courses of right IPS and the whole brain within the GM mask. Then, the Fisher's z conversion was executed for subsequent analysis. 44
2.8. Statistical analysis
Statistical Package for Social Sciences (SPSS) software (version 26) was used to perform statistical analysis. In demographic and neuropsychological characteristics, continuous variables were expressed as mean ± standard deviation (SD), and discontinuous variables were expressed as median ± interquartile range (IQR). Kolmogorov–Smirnov test (n > 50) or Shapiro–Wilk test (n ≤ 50) was used to evaluate the normality hypothesis of the data. The one‐way analysis of variance (ANOVA) was used to analyze the differences of continuous variables with normal distribution among the three groups, and Kruskal–Wallis H‐test was used to analyze continuous variables without normal distribution. The post hoc test was obtained by two‐sample t‐test Bonferroni correction or Mann‐Whitney U‐test (p < 0.05). The chi‐square test was used for the difference in gender among the three groups.
Using DPABI software, the ANOVA test within the DAN mask was to compare the differences in fALFF, ReHo, and GMV among these three groups, the age, gender, and years of education as covariates. The nonparametric permutation test (1000 permutations) was conducted, and the significance level was set to p < 0.05. Post hoc comparisons using the two‐sample t‐test with the mask resulted from ANOVA analyses were performed after controlling the effects of age, gender, and years of education. The significance level was set with TFCE‐FWE corrected at p < 0.05 in fALFF and ReHo, and TFCE‐FDR corrected at p < 0.05 in GMV.
A one‐way ANOVA analysis within GM mask was conducted using DPABI to compare the differences in FC across three groups, including aMCI, SCD, and HCs, with age, gender, and years of education as covariates. The nonparametric permutation test (1000 permutations) was conducted, and the significance level was set to p < 0.05. The two‐sample t‐test was used for post hoc comparisons with the mask resulting from ANOVA analyses after controlling the effects of age, gender, and years of education. The TFCE‐FDR correction was applied with a threshold of p < 0.05 in FC.
The Pearson correlation analysis was conducted to explore the relationship between functional and structural alterations and cognitive performance after controlling the effects of age, gender, and years of education in the SPSS software. Moreover, we evaluated the sensitivity and specificity of all meaningful biomarkers in combination to predict their value in SCD and aMCI with ROC curves.
3. RESULTS
3.1. Demographic and neuropsychological characteristics
Table 1 summarizes the demographic and neuropsychological characteristics of 125 participants in three groups, including 42 HCs (mean age 63.82 ± 7.35), 43 SCD (mean age 63.63 ± 7.10), and 40 aMCI (mean age 64.65 ± 7.99). As is expected, the results showed significant differences in cognitive performance. Compared with HCs and SCD, the aMCI group showed lower MMSE, MoCA, and MDRS‐2 (Bonferroni corrected for post hoc, all p < 0.05). At the same time, the aMCI group also showed significantly lower EM, EF, and IPS (Bonferroni corrected for post hoc, all p < 0.05) than HCs and SCD.
3.2. FALFF, ReHo, and FC alterations of DAN in SCD and aMCI
The fALFF ANOVA analysis had significant differences in some brain regions among HCs, SCD, and aMCI groups, including the left IOG, left inferior parietal lobule (IPL), and left preCG. Compared to HCs, the SCD group displayed increased alterations in left preCG and left IOG and decreased changes in the left IPL, respectively. In comparison with aMCI, increased fALFF values in the left IOG and left preCG were significant in the SCD group (see Table 2 and Figure 1).
TABLE 2.
FALFF values across HCs, SCD, and aMCI groups.
| Region (aal) | Peak MNI coordinate | F/t | Cluster number | ||
|---|---|---|---|---|---|
| x | y | z | |||
| ANOVA | |||||
| L inferior occipital gyrus | −51 | −69 | −18 | 8.4755 | 31 |
| L inferior parietal lobule | −30 | −75 | 48 | 6.248 | 30 |
| L precentral gyrus | −54 | −3 | 30 | 11.0853 | 20 |
| SCD > HCs | |||||
| L precentral gyrus | −54 | −3 | 30 | 4.1092 | 15 |
| L inferior occipital gyrus | −48 | −69 | −12 | 2.6455 | 9 |
| SCD < HCs | |||||
| L inferior parietal lobule | −30 | −75 | 48 | −3.0031 | 26 |
| SCD > aMCI | |||||
| L inferior occipital gyrus | −51 | −69 | −18 | 3.8032 | 21 |
| L precentral gyrus | −54 | −3 | 30 | 3.5988 | 15 |
Note: Brain regions showed significant differences in fALFF of the DAN across three groups (TFCE‐FWE corrected, cluster size >15 voxels, p < 0.05) and results of post hoc analysis in voxel‐wise analysis (Bonferroni corrected, cluster size >5 voxels, p < 0.05).
Abbreviations: aMCI, amnestic mild cognitive impairment; HCs, healthy controls; L, left; R, right; SCD, subjective cognitive decline.
FIGURE 1.
Brain regions showed significant differences in fALFF of the DAN across three groups. aMCI, amnestic mild cognitive impairment; HCs, healthy controls; IOG, inferior occipital gyrus; IPL, inferior parietal lobule; L, left; preCG, precentral gyrus; R, right; SCD, subjective cognitive decline.
In the ReHo ANOVA analysis, significant results were mainly in the right MFG, right MTG, and left IOG among three groups. Compared to HCs, the SCD and aMCI groups showed decreased ReHo values in the right MTG. Incredibly, there was an increase in the SCD group in the left IOG, and right ITG compared to HCs. Besides, the aMCI group exhibited significantly increased ReHo values in the right MTG and decreased in the left IOG in comparison with SCD (see Table 3 and Figure 2).
TABLE 3.
ReHo values across HCs, SCD, and aMCI groups.
| Region (aal) | Peak MNI coordinate | F/t | Cluster number | ||
|---|---|---|---|---|---|
| x | y | z | |||
| ANOVA | |||||
| R middle frontal gyrus | 54 | 24 | 39 | 0.12798 | 227 |
| R middle temporal gyrus | 60 | −66 | 15 | 7.7837 | 145 |
| L inferior occipital gyrus | −54 | −69 | −12 | 8.1143 | 49 |
| SCD > HCs | |||||
| L inferior occipital gyrus | −48 | −69 | −12 | 3.253 | 43 |
| R inferior temporal gyrus | 51 | −63 | −3 | 3.3779 | 33 |
| SCD < HCs | |||||
| R middle temporal gyrus | 60 | −66 | 15 | −3.436 | 71 |
| aMCI < HCs | |||||
| R middle temporal gyrus | 60 | −63 | 15 | −2.6433 | 15 |
| SCD > aMCI | |||||
| L inferior occipital gyrus | −54 | −69 | −12 | 4.1794 | 48 |
| SCD < aMCI | |||||
| R middle temporal gyrus | 57 | −51 | 12 | −3.2725 | 54 |
Note: Brain regions showed significant differences in ReHo of the DAN across three groups (TFCE‐FWE corrected, cluster size >40 voxels, p < 0.05) and results of post hoc analysis in voxel‐wise analysis (Bonferroni corrected, cluster size >10 voxels, p < 0.05).
Abbreviations: aMCI, amnestic mild cognitive impairment; HCs, healthy controls; L, left; R, right; SCD, subjective cognitive decline.
FIGURE 2.

Brain regions showed significant differences in ReHo of the DAN across three groups. aMCI, amnestic mild cognitive impairment; HCs, healthy controls; IOG, inferior occipital gyrus; L, left; MFG, middle frontal gyrus; MTG, middle temporal gyrus; R, right; SCD, subjective cognitive decline.
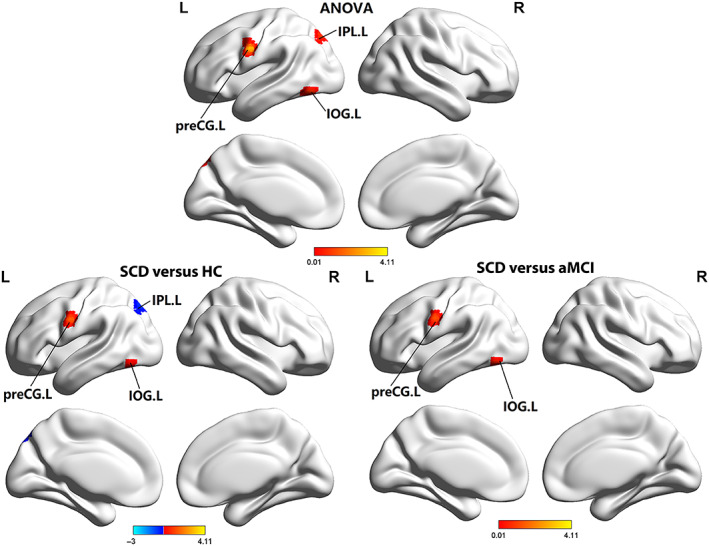
The ANOVA analysis showed significant alterations across these three groups concerning the FC aspect, especially in the left IPL. Compared to HCs, increased FC values in the SCD group were significant in the left IPL. The aMCI group showed significantly decreased FC values in the left IPL (see Table 4 and Figure 3).
TABLE 4.
FC values across HCs, SCD, and aMCI groups.
| Region (aal) | Peak MNI coordinate | F/t | Cluster number | ||
|---|---|---|---|---|---|
| x | y | z | |||
| ANOVA | |||||
| L inferior parietal lobule | −30 | −57 | 48 | 16.0974 | 76 |
| L inferior parietal lobule | −42 | −51 | 33 | 12.4807 | 56 |
| SCD > HCs | |||||
| L inferior parietal lobule | −30 | −57 | 48 | 4.0238 | 39 |
| SCD > aMCI | |||||
| L inferior parietal lobule | −30 | −57 | 51 | 5.0537 | 76 |
| L inferior parietal lobule | −42 | −45 | 48 | 4.3787 | 56 |
Note: Brain regions showed significant differences in FC of the DAN across three groups (TFCE‐FDR corrected, cluster size >50 voxels, p < 0.05) and results of post hoc analysis in voxel‐wise analysis (Bonferroni corrected, cluster size >30 voxels, p < 0.05).
Abbreviations: aMCI, amnestic mild cognitive impairment; HCs, healthy controls; L, left; R, right; SCD, subjective cognitive decline.
FIGURE 3.
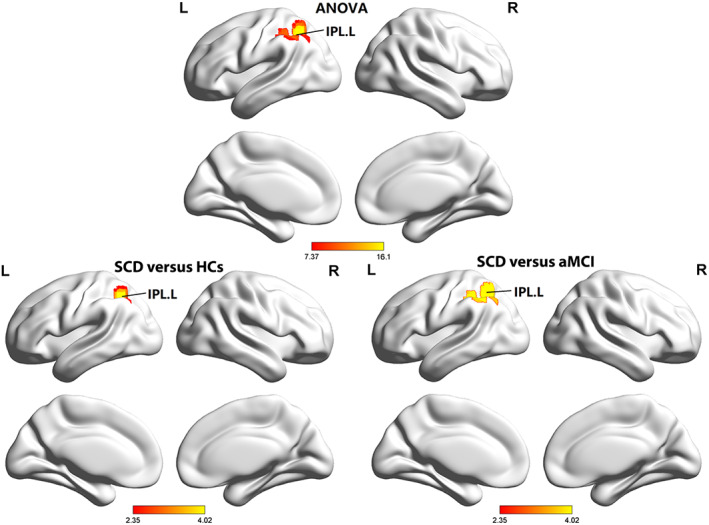
Brain regions showed significant differences in FC of the DAN across three groups. aMCI, amnestic mild cognitive impairment; HCs, healthy controls; IPL, inferior parietal lobule; L, left; R, right; SCD, subjective cognitive decline.
3.3. GMV and cortical thickness alterations of DAN in SCD and aMCI
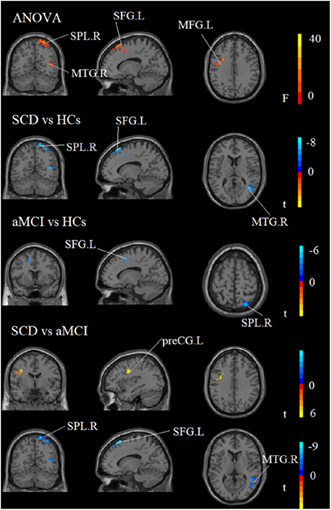
The ANOVA analysis showed significant changes in GMV of DAN across three groups, including the left MFG, right SPL, right MTG, and left SFG. Compared to HCs, the SCD and aMCI groups showed decreased GMV values in the left SFG and right SPL. The aMCI group exhibited an increase in the right MTG, left SFG, and right SPL and a decrease in the left preCG compared to SCD (see Table 5 and Figure 4).
TABLE 5.
GMV values across HCs, SCD, and aMCI groups.
| Region (aal) | Peak MNI coordinate | F/t | Cluster number | ||
|---|---|---|---|---|---|
| x | y | z | |||
| ANOVA | |||||
| L middle frontal gyrus | −30 | 9 | 33 | 26.1047 | 143 |
| R superior parietal lobule | 24 | −60 | 72 | 30.7415 | 112 |
| R middle temporal gyrus | 39 | −60 | 12 | 27.6392 | 88 |
| L superior frontal gyrus | −15 | 24 | 66 | 38.0666 | 85 |
| SCD < HCs | |||||
| R middle temporal gyrus | 39 | −60 | 12 | −7.7543 | 40 |
| L superior frontal gyrus | −15 | 24 | 66 | −7.2529 | 35 |
| R superior parietal lobule | 15 | −60 | 75 | −5.7876 | 24 |
| aMCI < HCs | |||||
| L superior frontal gyrus | −15 | 9 | 51 | −4.8495 | 43 |
| R superior parietal lobule | 24 | −69 | 63 | −5.1014 | 30 |
| SCD < aMCI | |||||
| R middle temporal gyrus | 39 | −54 | 9 | −4.8384 | 72 |
| R superior parietal lobule | 15 | −60 | 75 | −5.9125 | 57 |
| L superior frontal gyrus | −15 | 24 | 66 | −8.7607 | 43 |
| SCD > aMCI | |||||
| L precentral gyrus | −36 | −3 | 33 | 5.8708 | 44 |
Note: Brain regions showed significant differences in GMV of the DAN across three groups (TFCE‐FDR corrected, cluster size >80 voxels, p < 0.05) and results of post hoc analysis in voxel‐wise analysis (Bonferroni corrected, cluster size >20 voxels, p < 0.05).
Abbreviations: aMCI, amnestic mild cognitive impairment; HCs, healthy controls; L, left; R, right; SCD, subjective cognitive decline.
FIGURE 4.
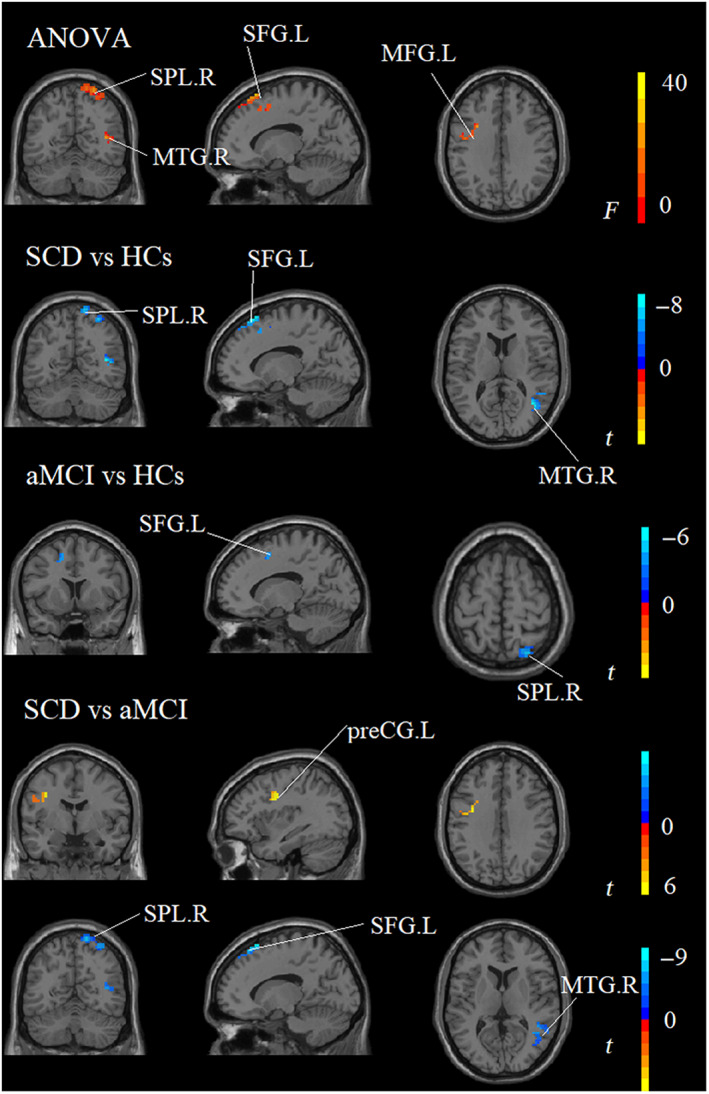
Brain regions showed significant differences in GMV of the DAN across three groups. aMCI, amnestic mild cognitive impairment; HCs, healthy controls; L, left; MFG, middle frontal gyrus; MTG, middle temporal gyrus; R, right; SCD, subjective cognitive decline; SFG, superior frontal gyrus; SPL, superior parietal lobule.
Regarding cortical thickness, the ANOVA analysis showed significant differences across these three groups in the right ITG. Compared to HCs, decreased signal in the right ITG was particularly in the SCD group (Bonferroni corrected for post hoc, p < 0.05) (see Table 6).
TABLE 6.
Cortical thickness across HCs, SCD, and aMCI groups.
| Cortical thickness | HCs | SCD | aMCI | F | p |
|---|---|---|---|---|---|
| L Superior parietal lobule | 2.198 (0.113) | 2.195 (0.128) | 2.192 (0.141) | 0.024 | 0.976 |
| R Superior parietal lobule | 2.174 (0.103) | 2.165 (0.136) | 2.172 (0.140) | 0.063 | 0.939 |
| L Inferior parietal lobule | 2.466 (0.119) | 2.434 (0.105) | 2.449 (0.144) | 0.728 | 0.485 |
| R Inferior parietal lobule | 2.465 (0.117) | 2.444 (0.110) | 2.449 (0.143) | 0.329 | 0.721 |
| L Middle temporal gyrus | 2.749 (0.103) | 2.741 (0.129) | 2.778 (0.143) | 0.952 | 0.389 |
| R Middle temporal gyrus | 2.815 (0.120) | 2.813 (0.129) | 2.822 (0.129) | 0.058 | 0.944 |
| L Inferior temporal gyrus | 2.889 (0.096) | 2.835 (0.159) | 2.854 (0.149) | 1.670 | 0.192 |
| R Inferior temporal gyrus | 2.880 (0.129) | 2.803 (0.126) | 2.866 (0.144) | 4.040 | 0.020 a |
| Precentral | 2.529 (0.140) | 2.556 (0.173) | 2.505 (0.206) | 0.895 | 0.411 |
| R Precentral | 2.508 (0.141) | 2.527 (0.172) | 2.467 (0.193) | 1.311 | 0.273 |
Note: Numbers are given as means (standard deviation, SD) unless stated otherwise. Brain regions showed significant differences in the cortical thickness of the DAN across three groups (all p < 0.05) and results of post hoc analysis (Bonferroni corrected, p < 0.05).
Abbreviations: aMCI, amnestic mild cognitive impairment; HCs, healthy controls; L, left; R, right; SCD, subjective cognitive decline.
Post hoc analysis showed a significant difference between SCD and HCs.
3.4. Pearson correlation analysis
Pearson correlation analysis was conducted between functional and structural alterations in DAN and neuropsychological scales (Figure 5, all p < 0.05). This study mainly focused on analyzing HCs, SCD, and aMCI groups. The analysis showed that the fALFF value in the left IOG was positively correlated with EM, EF, and IPS scores (r = 0.208, p = 0.020; r = 0.370, p = 0.000; r = 0.239, p = 0.007). The ReHo value in the left IOG and right MFG were also positively correlated with the EF score (r = 0.284, p = 0.001) and IPS score (r = 0.200, p = 0.025). Moreover, the altered FC in the left IPL was positively correlated with IPS score (r = 0.287, p = 0.001). The GMV value in the left MFG was positively correlated with EM and EF (r = 0.223, p = 0.012; r = 0.209, p = 0.019). The cortical thickness value in the right ITG was positively correlated with IPS (r = 0.216, p = 0.015).
FIGURE 5.
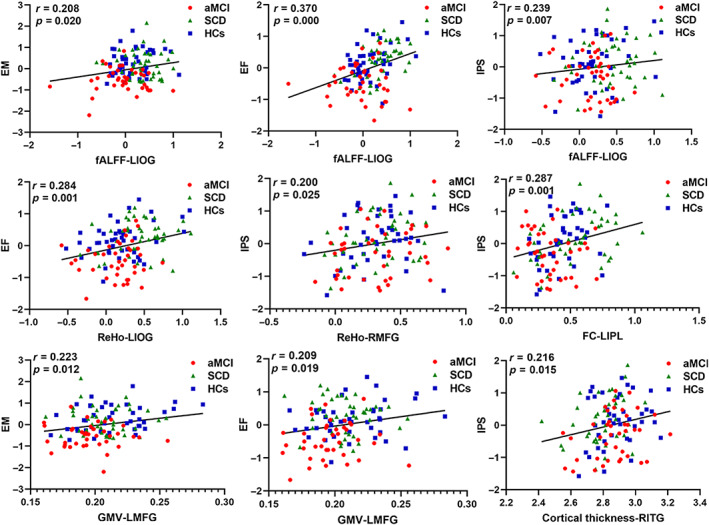
Pearson correction analysis between cognitive performance and functional and structural alterations of DAN in HCs, SCD, and aMCI groups. aMCI, amnestic mild cognitive impairment; fALFF, fractional amplitude of low‐frequency fluctuation; FC, functional connectivity; GMV, gray matter volume; HCs, healthy controls; IOG, inferior occipital gyrus; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; L, left; MFG, middle frontal gyrus; R, right; ReHo, regional homogeneity; SCD, subjective cognitive decline.
3.5. ROC analysis
In the groups of SCD and HCs, the area under the curve (AUC) values of fALFF in the left IPL and ReHo in the right MTG were 0.744 and 0.692. The GMV in the right MTG, left SFG, and right SPL had the AUC values of 0.815, 0.823, and 0.761. The AUC values of cortical thickness in the right ITG were 0.636 (all p < 0.05, Figure 6A).
FIGURE 6.
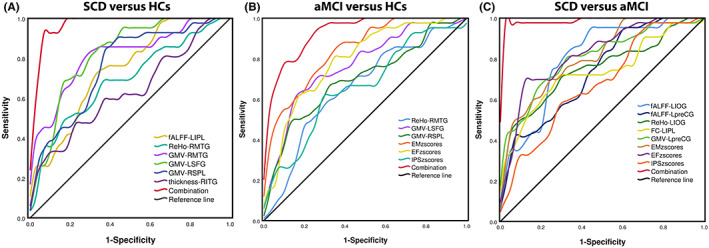
Classification of individuals across three groups on ROC analysis. (A) ROC curve presenting the classification of SCD vs. HCs; (B) ROC curve presenting the classification of aMCI vs. HCs; (C) ROC curve presenting the classification of SCD vs. aMCI; aMCI, amnestic mild cognitive impairment; HCs, healthy controls; L, left; R, right; SCD, subjective cognitive decline.
In the groups of aMCI and HCs, the AUC values of ReHo in the right MTG and GMV in the left SFG and right SPL were 0.658, 0.770 and 0.699. The EM, EF, and IPS had the AUC values of 0.840, 0.791, and 0.660 (all p < 0.05, Figure 6B).
In the groups of SCD and aMCI, the AUC values of fALFF in the left IOG and left preCG were 0.802 and 0.737. The AUC values of ReHo in the left IOG and altered FC in the left IPL were 0.751 and 0.763. The EM, EF, and IPS had the AUC values of 0.810, 0.824, and 0.677 (all p < 0.05, Figure 6C).
4. DISCUSSION
This study investigated functional and structural alterations of DAN in SCD and aMCI groups comprehensively. According to our findings, fALFF, ReHo, FC, GMV, and cortical thickness had significant changes. Furthermore, the combined multiple indicators of DAN can be acted as the latent neuroimaging markers of preclinical and early‐stage AD for their high diagnostic value.
4.1. Altered functional patterns of DAN in SCD and aMCI
As presented, fALFF alterations were mainly located in the left IOG, left IPL, and left preCG. As we all know, IOG is associated with cognitive performance, with a previous PET study showing a significant increase in metabolism in the occipital cortex in cognitive impairment. 45 A previous study reported that the SCD group exhibited higher fALFF values than HCs, consistent with our study. 46 The function of preCG is to start the purposeful movement by integrating the information through the sensory‐motor cortex. 47 Increased fALFF values indicated enhanced neuronal connectivity in the left IOG and left preCG in the process of cognitive impairment. 48 IPL has a close connection with episodic memory. 49 Compared to HCs, the SCD group exhibited a significant decrease in fALFF values in the IPL, further confirming the continuous episodic memory decline from HCs to SCD. 50
With regard to ReHo values, right MTG and left IOG were shown significant alterations. Previous studies had found that the MTG was crucial in many cognitive domains such as language processing and deductive reasoning. 51 Compared to HCs, the SCD group showed lower ReHo values, which meant a greater deficit in cognitive performance. 50 The ReHo values in the left IOG showed increased alterations, which was the same as the fALFF. The increased value indicated that the neuronal activity in the local brain area tended to increase. 52 A study reported that neurodegeneration caused the accumulation of amyloid plaques to overexcite neurons. 53 This research could suggest that the increased ReHo in the left IOG may play an essential role in early functional compensation.
As for FC, the most apparent change was mainly in the left IPL. IPL integrates information from different sensory modalities and plays an essential role in various higher cognitive functions. 54 Compared to HCs and aMCI, the SCD group showed increased FC in the left IPL. IPL was the classic brain region of the DAN. The increased connectivity in left IPL was within the DAN, which meant the increased activity of the DAN itself to some extent. 55
4.2. Altered structural patterns of DAN in SCD and aMCI
Regarding the GMV values, the reduction in the SCD and aMCI groups was mainly in the left MFG, right SPL, right MTG, and left SFG. A study reported that the GMV of MFG was significantly correlated with memory performance. 56 The decreased GMV in MFG can affect the processing of word understanding in the elderly. 57 SPL has also been related to processing movement in space, and the GMV in SPL declined with the progression of AD. 58 Surprisingly, a study reported that those participants who spent more time‐performing sports activity per week showed higher GMV in SPL. 59 In this way, we can prevent AD progression through sports activity. The research informed that the GMV showed a noticeable decline in the MTG. 56 The subregions of MTG are related to human episodic memory. 51 A study also indicated that primary foci of atrophy were identified in the MTG in AD patients. 60 Thus, the atrophy of MTG could be used as a predictive factor for AD progression. The SFG has been associated with fluency tasks. Especially in aMCI, the SFG predicted damaged memory performance in both category and design fluency. 61
The cortical thickness of right ITG between SCD and HCs showed a significant decrease. The ITG plays an essential role in verbal fluency. Individuals with cognitive impairment had significantly fewer synapses in the ITG than individuals with no cognitive impairment. These results demonstrated that the ITG was affected during the early stage of the disease and may underlie some of the early AD‐related clinical dysfunctions.
4.3. Cognitive performance and alterations of DAN in SCD and aMCI
In terms of cognitive performance, Pearson correlation analysis showed that the decreased fALFF values in the left IOG were accompanied by decreased EM, EF, and IPS. At the same time, decreased ReHo in the left IOG also had a relationship with decreased EF. The occipital lobe lesions are often associated with visuospatial‐structural dysfunction, usually occurring in the early stages of AD. The study showed that the functional value in the occipital lobe was reduced, related to memory loss. 62 The positive relation between the cognitive performance and FC in the left IPL was clear for its role in cognitive processes. 63 The decreased GMV in preCG in SCD and aMCI was related to cognitive dysfunction. Voxel‐level GMV analysis revealed strong relationships between volumes of the preCG and olfactory identification impairments, which frequently occurred in the AD disease. 64 The results demonstrated the involvement of DAN in cognitive function, consistent with previous studies. 65 , 66 Moreover, it further confirmed the functional and structural alterations in DAN had a significant connection with cognitive function.
Through the ROC analysis, the most important finding in this study was that the neuroimages of DAN across the three groups had a very high accuracy. The AUC values of GMV in the left SFG and right SPL were up to 0.815 (sensitivity = 85.7%, specificity = 67.4%) and 0.823 (sensitivity = 90.5%, specificity = 58.1%) in SCD and HCs. The AUC value of EM in aMCI and HCs was 0.84 (sensitivity = 83.3%, specificity = 67.5%) in aMCI and HCs. The AUC values of left IOG, EM, and EF were 0.802 (sensitivity = 95.3%, specificity = 57.5%), 0.810 (sensitivity = 69.8%, specificity = 77.5%), and 0.824 (sensitivity = 69.8%, specificity = 90%) in SCD and aMCI. All results showed the advantages of the functional and structural alterations of DAN in studying preclinical and early‐stage AD and provided a new sight for the diagnosis and differentiation in the progression of AD.
4.4. Limitations
There were the following notable limitations in this study. First of all, we had a small sample size, and only 42 HCs, 43 SCD, and 40 aMCI were included in our study. At the same time, our NBH‐ADsnp‐2 database is being expanded, and more volunteers are being recruited. Therefore, we will further fill the gap of a small sample size. Secondly, this study was just a baseline study. A longitudinal study with further follow‐up of these data is required to evaluate the alterations of neuroimaging markers in AD progression comprehensively. Moreover, the gender distribution in these three groups was unbalanced, which may lead to bias in our results. Fortunately, we took age, gender, and education as covariates in the process of statistical analyses in order to reduce the impact to a certain extent. Lastly, the plasma biomarker of early‐stage AD spectrum plays an important role in clinical diagnosis. In the future, we will pay more attention to the plasma biomarker to expand the significance of research.
5. CONCLUSION
Our current study demonstrated obviously functional and structural alterations of DAN in SCD and aMCI. We found that the abnormalities of the functional alterations were especially located in the IPL, IOG, and MTG, whereas structural alterations were mainly in the SPL and ITG. Furthermore, cognitive performance was closely related to these significant alterations. Our study further suggested that the combined multiple indicators of DAN could be acted as the latent neuroimaging markers of preclinical and early‐stage AD for their high diagnostic value.
AUTHOR CONTRIBUTIONS
HW, YS, XY, XL, and JC: designed the study. HW, YS, XY, CS, HG, ZY, WQ, QY, and XL: collected the data. HW, YS, and XY: analyzed the data and prepared the manuscript. XL and JC modified the article and approved the submission. HW, YS, and XY contributed equally (joint first authors).
CONFLICT OF INTEREST
The authors declare that they have no competing financial interests.
Supporting information
Appendix S1–S5
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81701675); the Key Research and Development Plan (Social Development) Project of Jiangsu Province (No. BE2018608 and BE2022679); Natural Science Foundation of Jiangsu Basic Research Program (No. BK20221185); Special Funded Project of Nanjing Drum Tower Hospital (No. RC2022‐023).
Wu H, Song Y, Yang X, et al. Functional and structural alterations of dorsal attention network in preclinical and early‐stage Alzheimer's disease. CNS Neurosci Ther. 2023;29:1512‐1524. doi: 10.1111/cns.14092
Huimin Wu, Yu Song, and Xinyi Yang contributed equally to this work.
Contributor Information
Xingjian Lin, Email: linxingjian@njmu.edu.cn.
Jiu Chen, Email: ericcst@aliyun.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13:369‐396. [DOI] [PubMed] [Google Scholar]
- 4. Liu P, Wu L, Peng G, et al. Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019;80:633‐643. [DOI] [PubMed] [Google Scholar]
- 5. Hao L, Xing Y, Li X, et al. Risk factors and neuropsychological assessments of subjective cognitive decline (plus) in Chinese memory clinic. Front Neurosci. 2019;13:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perry RJ, Watson P, Hodges JR. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer's disease: relationship to episodic and semantic memory impairment. Neuropsychologia. 2000;38(3):252‐271. [DOI] [PubMed] [Google Scholar]
- 8. Redel P, Bublak P, Sorg C, et al. Deficits of spatial and task‐related attentional selection in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2012;33(1):195.e27‐195.e42. [DOI] [PubMed] [Google Scholar]
- 9. Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). Am J Geriatr Psychiatry. 2005;13(2):134‐141. [DOI] [PubMed] [Google Scholar]
- 10. Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease. A critical review. Brain. 1999;122(Pt 3):383‐404. [DOI] [PubMed] [Google Scholar]
- 11. Liang J, Li Y, Liu H, et al. Increased intrinsic default‐mode network activity as a compensatory mechanism in aMCI: a resting‐state functional connectivity MRI study. Aging. 2020;12(7):5907‐5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17(12):777‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhan Y, Ma J, Alexander‐Bloch AF, et al. Longitudinal study of impaired intra‐ and inter‐network brain connectivity in subjects at high risk for Alzheimer's disease. J Alzheimers Dis. 2016;52(3):913‐927. [DOI] [PubMed] [Google Scholar]
- 14. Jiang R, Scheinost D, Zuo N, et al. A neuroimaging signature of cognitive aging from whole‐brain functional connectivity. Adv Sci. 2022;9:e2201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu H, Song Y, Chen S, et al. An activation likelihood estimation meta‐analysis of specific functional alterations in dorsal attention network in mild cognitive impairment. Front Neurosci. 2022;16:876568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suping Cai TC, Peng Y, Shen W, Li J, von Deneen KM, Huang L. Alzheimer's disease neuroimaging initiative, altered functional brain networks in amnestic mild cognitive impairment: a resting‐state fMRI study. Brain Imaging Behav. 2016;11:619‐631. doi: 10.1007/s11682-016-9539-0 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Zhao X, Xu S, et al. Using regional homogeneity to reveal altered spontaneous activity in patients with mild cognitive impairment. Biomed Res Int. 2015;2015:807093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balachandar R, John JP, Saini J, et al. A study of structural and functional connectivity in early Alzheimer's disease using rest fMRI and diffusion tensor imaging. Int J Geriatr Psychiatry. 2015;30(5):497‐504. [DOI] [PubMed] [Google Scholar]
- 19. Xue C, Sun H, Yue Y, et al. Structural and functional disruption of salience network in distinguishing subjective cognitive decline and amnestic mild cognitive impairment. ACS Chem Nerosci. 2021;12(8):1384‐1394. [DOI] [PubMed] [Google Scholar]
- 20. López‐Sanz D, Bruña R, Garcés P, et al. Functional connectivity disruption in subjective cognitive decline and mild cognitive impairment: a common pattern of alterations. Front Aging Neurosci. 2017;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei PH, Chen H, Ye Q, et al. Self‐reference network‐related interactions during the process of cognitive impairment in the early stages of Alzheimer's disease. Front Aging Neurosci. 2021;13:666437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roy M, Edde M, Fortier M, et al. A ketogenic intervention improves dorsal attention network functional and structural connectivity in mild cognitive impairment. Neurobiol Aging. 2022;115:77‐87. [DOI] [PubMed] [Google Scholar]
- 23. Wang S, Sun H, Hu G, et al. Altered insular subregional connectivity associated with cognitions for distinguishing the Spectrum of pre‐clinical Alzheimer's disease. Front Aging Neurosci. 2021;13:597455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen J, Ma N, Hu G, et al. rTMS modulates precuneus‐hippocampal subregion circuit in patients with subjective cognitive decline. Aging. 2020;13(1):1314‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan T, Wang W, Yang L, Chen K, Chen R, Han Y. Rich club disturbances of the human connectome from subjective cognitive decline to Alzheimer's disease. Theranostics. 2018;8(12):3237‐3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cedres N, Machado A, Molina Y, et al. Subjective cognitive decline below and above the age of 60: a multivariate study on neuroimaging, cognitive, clinical, and demographic measures. J Alzheimers Dis. 2019;68(1):295‐309. [DOI] [PubMed] [Google Scholar]
- 27. Hao L, Wang X, Zhang L, et al. Prevalence, risk factors, and complaints screening tool exploration of subjective cognitive decline in a large cohort of the Chinese population. J Alzheimers Dis. 2017;60(2):371‐388. [DOI] [PubMed] [Google Scholar]
- 28. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303‐308. [DOI] [PubMed] [Google Scholar]
- 29. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256(3):240‐246. [DOI] [PubMed] [Google Scholar]
- 30. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting‐state) brain imaging. Neuroinformatics. 2016;14(3):339‐351. [DOI] [PubMed] [Google Scholar]
- 31. Chao‐Gan Y, Yu‐Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song Y, Wu H, Chen S, et al. Differential abnormality in functional connectivity density in preclinical and early‐stage Alzheimer's disease. Front Aging Neurosci. 2022;14:879836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang H, Zhao Y, Cao W, et al. Aberrant functional connectivity in resting state networks of ADHD patients revealed by independent component analysis. BMC Neurosci. 2020;21(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Avelar‐Pereira B, Bäckman L, Wåhlin A, Nyberg L, Salami A. Age‐related differences in dynamic interactions among default mode, frontoparietal control, and dorsal attention networks during resting‐state and interference resolution. Front Aging Neurosci. 2017;9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding JR, Zhu F, Hua B, et al. Presurgical localization and spatial shift of resting state networks in patients with brain metastases. Brain Imaging Behav. 2019;13(2):408‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baggio HC, Segura B, Sala‐Llonch R, et al. Cognitive impairment and resting‐state network connectivity in Parkinson's disease. Hum Brain Mapp. 2015;36(1):199‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lei X, Wang Y, Yuan H, Mantini D. Neuronal oscillations and functional interactions between resting state networks. Hum Brain Mapp. 2014;35(7):3517‐3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liang P, Li Z, Deshpande G, Wang Z, Hu X, Li K. Altered causal connectivity of resting state brain networks in amnesic MCI. PLoS One. 2014;9(3):e88476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi Q, Chen H, Jia Q, et al. Altered granger causal connectivity of resting‐state neural networks in patients with leukoaraiosis‐associated cognitive impairment‐a cross‐sectional study. Front Neurol. 2020;11:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang S, Rao J, Yue Y, et al. Altered frequency‐dependent brain activation and white matter integrity associated with cognition in characterizing preclinical Alzheimer's disease stages. Front Hum Neurosci. 2021;15:625232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan X, Han Y, Wei Y, et al. Regional homogeneity changes in amnestic mild cognitive impairment patients. Neurosci Lett. 2016;629:1‐8. [DOI] [PubMed] [Google Scholar]
- 42. Esposito R, Cieri F, Chiacchiaretta P, et al. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2018;12(1):127‐141. [DOI] [PubMed] [Google Scholar]
- 43. Lee D, Lee J, Namkoong K, Jung YC. Altered functional connectivity of the dorsal attention network among problematic social network users. Addict Behav. 2021;116:106823. [DOI] [PubMed] [Google Scholar]
- 44. Chen S, Song Y, Wu H, et al. Hyperconnectivity associated with anosognosia accelerating clinical progression in amnestic mild cognitive impairment. ACS Chem Nerosci. 2022;13(1):120‐133. [DOI] [PubMed] [Google Scholar]
- 45. Han Y, Lui S, Kuang W, Lang Q, Zou L, Jia J. Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One. 2012;7(2):e28664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun Y, Dai Z, Li Y, et al. Subjective cognitive decline: mapping functional and structural brain changes‐a combined resting‐state functional and structural MR imaging study. Radiology. 2016;281(1):185‐192. [DOI] [PubMed] [Google Scholar]
- 47. Taschereau‐Dumouchel V, Hétu S. Visuomotor representations within the human primary motor cortex: the elusive markers of visuomotor associative learning. J Neurosci. 2012;32:759‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. The influence of the amplitude of low‐frequency fluctuations on resting‐state functional connectivity. Front Hum Neurosci. 2013;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao Z, Lu J, Jia X, et al. Selective changes of resting‐state brain oscillations in aMCI: an fMRI study using ALFF. Biomed Res Int. 2014;2014:920902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Staffen W, Ladurner G, Höller Y, et al. Brain activation disturbance for target detection in patients with mild cognitive impairment: an fMRI study. Neurobiol Aging. 2012;33(5):1002.e1‐1002.e16. [DOI] [PubMed] [Google Scholar]
- 51. Berron D, van Westen D, Ossenkoppele R, Strandberg O, Hansson O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer's disease. Brain. 2020;143(4):1233‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cha J, Hwang JM, Jo HJ, Seo SW, Na DL, Lee JM. Assessment of functional characteristics of amnestic mild cognitive impairment and Alzheimer's disease using various methods of resting‐state FMRI analysis. Biomed Res Int. 2015;2015:907464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34(8):430‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430‐448. [DOI] [PubMed] [Google Scholar]
- 55. Wang P, Li R, Yu J, et al. Altered distant synchronization of background network in mild cognitive impairment during an executive function task. Front Behav Neurosci. 2017;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42(10):1313‐1335. [DOI] [PubMed] [Google Scholar]
- 57. Eckert MA, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. Age‐related effects on word recognition: reliance on cognitive control systems with structural declines in speech‐responsive cortex. J Assoc Res Otolaryngol. 2008;9(2):252‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Banaszkiewicz A, Bola Ł, Matuszewski J, et al. The role of the superior parietal lobule in lexical processing of sign language: insights from fMRI and TMS. Cortex. 2021;135:240‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eyme KM, Domin M, Gerlach FH, et al. Physically active life style is associated with increased grey matter brain volume in a medial parieto‐frontal network. Behav Brain Res. 2019;359:215‐222. [DOI] [PubMed] [Google Scholar]
- 60. Li X, Coyle D, Maguire L, Watson DR, McGinnity TM. Gray matter concentration and effective connectivity changes in Alzheimer's disease: a longitudinal structural MRI study. Neuroradiology. 2011;53(10):733‐748. [DOI] [PubMed] [Google Scholar]
- 61. Peter J, Kaiser J, Landerer V, et al. Category and design fluency in mild cognitive impairment: performance, strategy use, and neural correlates. Neuropsychologia. 2016;93(Pt A):21‐29. [DOI] [PubMed] [Google Scholar]
- 62. Min J, Zhou XX, Zhou F, Tan Y, Wang WD. A study on changes of the resting‐state brain function network in patients with amnestic mild cognitive impairment. Braz J Med Biol Res. 2019;52(5):e8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Greene SJ, Killiany RJ. Subregions of the inferior parietal lobule are affected in the progression to Alzheimer's disease. Neurobiol Aging. 2010;31(8):1304‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu X, Geng Z, Zhou S, et al. Brain structural correlates of odor identification in mild cognitive impairment and Alzheimer's disease revealed by magnetic resonance imaging and a Chinese olfactory identification test. Front Neurosci. 2019;13:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qian S, Zhang Z, Li B, Sun G. Functional‐structural degeneration in dorsal and ventral attention systems for Alzheimer's disease, amnestic mild cognitive impairment. Brain Imaging Behav. 2015;9(4):790‐800. [DOI] [PubMed] [Google Scholar]
- 66. Zhang Z, Zheng H, Liang K, et al. Functional degeneration in dorsal and ventral attention systems in amnestic mild cognitive impairment and Alzheimer's disease: an fMRI study. Neurosci Lett. 2015;585:160‐165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1–S5
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


