Abstract
Objectives
This study aimed to explore the incidence and risk factors for emergence agitation (EA) in elderly patients who underwent total joint arthroplasty (TJA) under general anaesthesia, and to assess their predictive values.
Design
Single-centre retrospective cohort study.
Setting
A 1600-bed general tertiary hospital in China.
Participants
This study enrolled 421 elderly patients scheduled for elective primary TJA under general anaesthesia.
Primary and secondary outcome measures
EA was assessed using the Richmond Agitation Sedation Scale during the awakening period after surgery in the post-anaesthesia care unit. Risk factors for EA were identified using univariate and multivariable logistic analyses. The receiver operating characteristic (ROC) curve was used to assess the predictive values of the risk factors for EA.
Results
The incidence of EA in elderly patients who underwent TJA was 37.6%. According to the multivariable logistic analysis, postoperative pain (95% CI: 1.951 to 3.196), male sex (95% CI: 1.781 to 6.435), catheter-related bladder discomfort (CRBD) (95% CI: 4.001 to 15.392) and longer fasting times for solids (95% CI: 1.260 to 2.301) and fluids (95% CI: 1.263 to 2.365) were independent risk factors for EA. As shown by the ROC analysis, postoperative pain and fasting times for solids and fluids had good predictive values, with areas under the ROC curve equalling 0.769, 0.753 and 0.768, respectively.
Conclusions
EA is a common complication after TJA in elderly patients. Some risk factors, including postoperative pain, male sex, CRBD and longer fasting times, can increase the incidence of EA. These risk factors may contribute to identifying high-risk patients, which facilitates the development of effective strategies to prevent and treat EA.
Trial registration number
ChiCTR1800020193.
Keywords: anaesthesia in orthopaedics, adult anaesthesia, adult orthopaedics, knee, hip
STRENGTHS AND LIMITATIONS OF THIS STUDY.
In this study, the medical records of 421 patients who underwent total joint arthroplasty were reviewed. Univariate and multivariable logistic analyses were used to identify the risk factors of emergence agitation, and the receiver operating characteristic curve was used to evaluate the predictive values of the risk factors.
This work was a single-centre retrospective study, and the generalizability of the results is weak.
Only patients with one category of arthritis were studied.
Introduction
Emergence agitation (EA), a common complication during the awakening period after general anaesthesia, refers to a temporary state of mental and motor excitement.1 Clinical features of EA include disorientation, excitation, agitation and combative behaviours.2 3 The incidence of EA in adults varies from 4.7% to 74%.4 EA can also increase the risk of wound bleeding or dehiscence, self-extubation, falling out of bed and violent behaviour towards staff.5 It may also prolong the patient’s stay in the post-anaesthesia care unit (PACU) and increase the demand for medical staff, resulting in higher medical costs.6 Elderly individuals are one of the main population groups affected by EA.7 Cardiovascular and cerebrovascular diseases are common in elderly individuals.8 Thus, EA may have more serious adverse consequences for elderly patients.5
Total joint arthroplasty (TJA) is a successful treatment protocol for end-stage knee and hip osteoarthritis (OA).9 Annually, more than 1 million people undergo TJA in the USA.10 As the population ages, the demand for TJA surgery is expected to increase substantially in the coming years.11 Most patients suffer from moderate-to-severe pain after TJA,12 which is one of the risk factors for EA in adult patients.3 13 14 The incidence and risk factors for EA in adults vary depending on the surgery15–17; however, reports on the incidence and risk factors for EA after TJA are lacking.
In this study, we retrospectively collected the medical records of 421 elderly patients who underwent general anaesthesia for TJA. We aimed to determine the incidence and risk factors of postoperative EA in elderly patients, to assess the predictive values, and provide guidance for preventing and treating EA.
Materials and methods
The trial was registered in the Chinese Clinical Registry (ChiCTR1800020193).
Patients
We enrolled 421 patients who underwent TJA under general anaesthesia at our hospital from December 2019 to June 2021. Inclusion criteria included (1) preoperative OA diagnosis, (2) age ≥60 years, (3) American Society of Anesthesiologists (ASA) physical status I–III and (4) having undergone scheduled elective primary TJA under general anaesthesia. Patients with any of the following conditions were excluded: revision TJA, spinal or epidural anaesthesia, general anaesthesia within the past 6 months and preoperative diagnosis of neuropsychiatric disorder.
Routine practice of perioperative management
Anaesthesia was induced with intravenous midazolam, etomidate, sufentanil and rocuronium. Tracheal intubation was completed after 2 min. Ultrasound-guided femoral nerve block (FNB) was performed in patients undergoing total knee arthroplasty (TKA), while ultrasound-guided fascia iliac compartment block (FICB) was performed in patients undergoing total hip arthroplasty (THA). All 20 mL (0.5%) ropivacaine solutions were infused into the nerve block. Urinary catheterisation was performed in all patients after inducing anaesthesia. Anaesthesia was maintained using intravenous remifentanil and propofol. Patients were transferred to the PACU after the operation. These patients were extubated in the PACU.
Specialty nurses assessed all patients in the PACU using a standardised protocol, including the Visual Analogue Scale (VAS), Richmond Agitation Sedation Scale (RASS) and Steward recovery scores. VAS was used to assess postoperative pain, and intravenous flurbiprofen was administered as an analgesic rescue when the VAS score was >4. EA was evaluated using the RASS,18 and table 1 presents the score criteria. Patients with a RASS score >1 were considered to have EA.18 Dexmedetomidine was administered in cases of severe agitation (RASS=4). Patients with Steward recovery scores >4 were transferred to the ward from the PACU.
Table 1.
Richmond Agitation Sedation Scale
| Score | Term | Description |
| +4 | Combative | Overtly combative, violent, immediate danger to staff |
| +3 | Very agitated | Pulls or removes tube(s) or catheter(s); aggressive |
| +2 | Agitated | Frequent non-purposeful movement; fights ventilator |
| +1 | Restless | Anxious but movements not aggressive or vigorous |
| 0 | Alert and calm | |
| −1 | Drowsy | Not fully alert but has sustained awakening (eye opening/eye contact) to voice (>10 s) |
| −2 | Light sedation | Briefly awake with eye contact to voice (<10 s) |
| −3 | Moderate sedation | Movement or eye opening to voice (but no eye contact) |
| −4 | Deep sedation | No response to voice, but movement or eye opening to physical stimulation |
| −5 | Unarousable | No response to voice or physical stimulation |
Scores of 1–4 indicated different levels of agitation, 0 indicated calmness and alertness, and −1 to −5 indicated different levels of sedation.
Data collection
The following patient-related variables were recorded: (1) population data and medical history, including age, sex, body mass index (BMI), ASA classification, education level, history of heart disease, respiratory disease, hypertension and diabetes; (2) perioperative clinical information, including operation type and times, body temperature after the surgery, VAS score, catheter-related bladder discomfort (CRBD), preoperative fasting times, intraoperative blood loss, warm treatment, postoperative nausea and vomiting, duration in PACU, RASS score and severe intraoperative hypotension (mean arterial pressure <65 mm Hg for at least 1 min); and (3) laboratory tests. Preoperative fasting time refers to the period from the last intake of liquids or solids to the beginning of anaesthesia induction.
Statistical analysis and sample size
The sample size was calculated using GPower software V.3.1 (Franz Faul, University of Kiel, Kiel, Germany). The effect size was set to 0.3, α level to 0.05 and 1−β to 0.85. A sample size of 100 patients was the optimal sample size needed to prove the difference between the two groups. Considering the easy acquisition of electronic medical records, we included patients who met the inclusion and exclusion criteria between December 2019 and June 2020.
Statistical analysis was performed using SPSS V.26.0. Continuous data were presented as means±SDs, and categorical data were presented as numbers and percentages. Independent risk factors were identified using univariate and multivariable logistic regression analyses. The measurement data were assessed for normal and non-normal distributions. Two independent sample t-tests were used to determine the differences between groups for continuous variables with a normal distribution. The non-parametric Mann-Whitney U test was used to compare differences between groups for continuous variables with non-normal distributions. Χ2 tests were used to determine differences between groups for categorical data. Variables with p<0.2 were entered in multivariable logistic regression analysis. A positive stepwise method was used to adjust for multiple risk factors. Each variable was expressed as an OR with a 95% CI. The predictive value of the risk factors for EA was assessed using the receiver operating characteristic (ROC) curve. The cut-off point was calculated based on the maximum Youden index value. Statistical significance was set at a p value of <0.05.
Patient and public involvement
None of the patients were involved in the design, data provision, analysis or publication of the study.
Results
General information on the study population
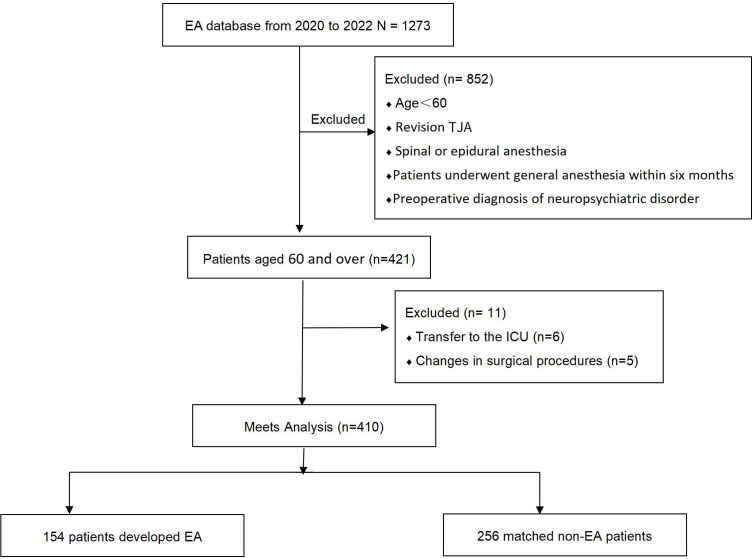
In total, 421 patients met the inclusion and exclusion criteria. However, 11 patients were excluded from the study: 6 were transferred to the intensive care unit (ICU) postoperatively and the surgical protocols of 5 patients were changed during the operation. Finally, the statistical analysis included 410 patients (figure 1). The incidence of EA was 37.6% (n=154) in 410 patients. All patients (n=410) were divided into two groups: EA and non-EA. Age, BMI, ASA classification, education level and medical history did not significantly differ between the two groups (table 2). The EA group had a significantly higher proportion of male patients than the non-EA group (p<0.05).
Figure 1.
Flow chart of study participants. In total, 421 patients met the inclusion and exclusion criteria. However, 11 patients were excluded from the study: 6 were transferred to the ICU postoperatively, and the surgical protocols of 5 were changed during the operation. Finally, the statistical analysis included 410 patients. EA, emergence agitation; ICU, intensive care unit; TJA, total joint arthroplasty.
Table 2.
Population data and medical history
| Variables | Agitation groups (n=154) | Non-agitation groups (n=256) | P value |
| Age | 69.84±6.53 | 69.39±6.82 | 0.238 |
| Male, n (%) | 91 (59.1) | 71 (27.7) | <0.001*** |
| BMI (kg/m2) | 22.75±4.31 | 23.17±2.56 | 0.253 |
| ASA classification, n (%) | 0.221 | ||
| I | 0 | 0 | |
| II | 118 (76.6) | 182 (71.1) | |
| III | 36 (23.4) | 74 (28.9) | |
| Education, n (%) | 0.412 | ||
| Illiteracy | 42 (27.3) | 55 (21.5) | |
| Primary school | 45 (29.2) | 93 (36.3) | |
| Secondary school | 59 (38.3) | 96 (37.5) | |
| University and above | 8 (5.2) | 12 (4.7) | |
| Medical history, n (%) | |||
| Heart disease | 0.816 | ||
| Yes | 72 (46.8) | 113 (44.1) | |
| No | 82 (53.2) | 143 (55.9) | |
| Respiratory diseases | 0.760 | ||
| Yes | 80 (51.9) | 129 (50.4) | |
| No | 74 (48.1) | 127 (49.6) | |
| Hypertension | 0.981 | ||
| Yes | 78 (50.6) | 131 (51.2) | |
| No | 76 (49.4) | 125 (48.8) | |
| Diabetes | |||
| Yes | 71 (46.1) | 119 (46.5) | 0.940 |
| No | 83 (53.9) | 137 (53.5) | |
Clinical information of patients was analysed using univariate analysis. Continuous data are presented as means±SDs, while categorical data are presented as numbers and percentages.
P value differences between patients in the two groups: *p<0. 05, ***p<0. 001.
ASA, American Society of Anesthesiologists; BMI, body mass index.
Perioperative clinical information and laboratory tests
Univariate analysis demonstrated significant differences between the EA and non-EA groups in the VAS score for postoperative pain, body temperature after the surgery, CRBD, preoperative fasting times and length of stay in the PACU.
Compared with the non-EA group, the VAS score was higher (p<0.05), body temperature after the surgery was lower (p<0.05), and the patient’s length of stay in the PACU and preoperative fasting times were longer in the EA group (p<0.05). Moreover, 77.3% (119 of 154) of patients in the EA group had CRBD, while 32.4% (83 of 256) of patients in the non-EA group experienced CRBD. This variable differed significantly between the two groups (p<0.05). Additionally, no significant differences were observed between the two groups regarding surgery type and times, intraoperative blood loss, intraoperative hypotension, warm treatment and laboratory tests (table 3).
Table 3.
Patients’ perioperative clinical information and agitation-related laboratory test indicators
| Variables | Agitation groups (n=154) |
Non-agitation groups (n=256) |
P value |
| Operation type, n (%) | 0.524 | ||
| TKA | 85 (55.2) | 133 (52.0) | |
| THA | 69 (44.8) | 123 (48.0) | |
| Operation times in TKA (min) | 144.42±59.96 | 143.91±46.19 | 0.236 |
| Operation times in THA (min) | 139.96±64.60 | 128.48±58.98 | 0.213 |
| VAS score for postoperative pain | 3.50±2.13 | 1.67±1.02 | <0.001*** |
| Body temperature at the end of the surgery (°C) | 35.87±0.73 | 36.03±0.94 | 0.037* |
| CRBD, n (%) | <0. 001*** | ||
| Yes | 119 (77.3) | 83 (32.4) | |
| No | 35 (22.7) | 173 (67.6) | |
| Preoperative fasting times (hours) | |||
| Fasting times for solids | 10.19±1.05 | 8.76±0.88 | <0.001*** |
| Fasting times for fluids | 4.81±1.14 | 2.99±0.92 | <0.001*** |
| Intraoperative blood loss (mL) | 217.26±30.18 | 200.32±27.48 | 0.224 |
| Severe intraoperative hypotension, n (%) | 0.261 | ||
| Yes | 14 (9.1) | 15 (5.9) | |
| No | 140 (90.9) | 241 (94.1) | |
| Postoperative nausea and vomiting, n (%) | 0.332 | ||
| Yes | 67 (43.5) | 124 (48.4) | |
| No | 87 (56.5) | 132 (51.6) | |
| The duration in PACU (min) | 32.83±14.07 | 31.00±8.57 | 0.025* |
| Warm treatment, n (%) | 0.880 | ||
| Yes | 68 (44.2) | 115 (44.9) | |
| No | 86 (55.8) | 141 (55.1) | |
| Laboratory testing | |||
| HCO3− (mmol/L) | 22.3±1.86 | 24.7±1.33 | 0.291 |
| PaCO2 (mm Hg) | 38.61±1.42 | 39.44±1.58 | 0.318 |
| PaO2 (mm Hg) | 89.52±1.74 | 90.17±1.55 | 0.282 |
| pH | 7.447±0.32 | 7.426±0.41 | 0.263 |
| Hb levels (g/L) | 16.6±1.93 | 17.1±1.85 | 0.274 |
Patients’ perioperative clinical information and agitation-related laboratory test indicators were analysed using univariate analysis. Continuous data are presented as means±SDs, while categorical data are presented as numbers and percentages.
P value differences between patients in the two groups: *p<0.05, ***p<0.001.
CRBD, catheter-related bladder discomfort; Hb, haemoglobin; HCO3−, bicarbonate ions in the blood; PaCO2, arterial CO2 pressure; PACU, post-anaesthesia care unit; PaO2, arterial oxygen pressure; THA, total hip arthroplasty; TKA, total knee arthroplasty; VAS, Visual Analogue Scale.
Multivariable logistic regression analysis
Based on the univariate analysis, variables included in the multivariable logistic regression analysis included the VAS score for postoperative pain, male sex, body temperature after the surgery, length of stay in the PACU, preoperative fasting times and CRBD.
The correlation between the VAS score for postoperative pain, male sex, preoperative fasting times, CRBD and EA after TJA could be determined based on multivariable logistic analysis (figure 2). The VAS score for postoperative pain (OR=2.497; 95% CI: 1.951 to 3.196), male sex (OR=3.391; 95% CI: 1.781 to 6.435), CRBD (OR=7.847; 95% CI: 4.001 to 15.392), fasting times for solids (OR=1.703; 95% CI: 1.260 to 2.301) and fasting times for fluids (OR=1.728; 95% CI: 1.263 to 2.365) were independent risk factors. However, we could not confirm the independence of variables, such as body temperature after the surgery and length of stay in the PACU, in the multivariable logistic analysis.
Figure 2.
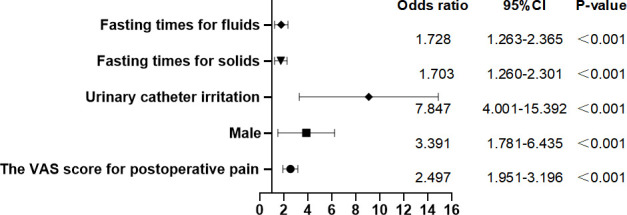
Risk factors for EA using meta-analysis plot. The VAS score for postoperative pain (OR=2.497; 95% CI: 1.951 to 3.196), male sex (OR=3.391; 95% CI: 1.781 to 6.435), urinary catheter irritation (OR=7.847; 95% CI: 4.001 to 15.392), fasting times for solids (OR=1.703; 95% CI: 1.260 to 2.301) and fasting times for fluids (OR=1.728; 95% CI: 1.263 to 2.365) were the independent risk factors. EA, emergence agitation; VAS, Visual Analogue Scale.
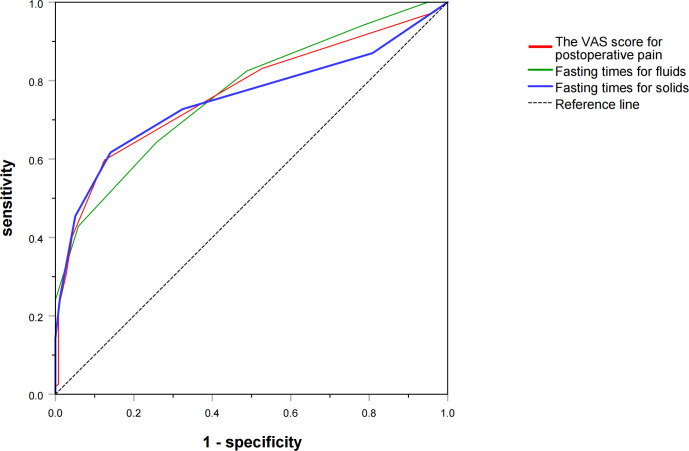
Results of ROC curves for risk factors
The predictive value analysed using the ROC curve is demonstrated in figure 3. The area under the ROC curve (AUC) for the VAS score was 0.769, with a cut-off value of 4.0, sensitivity of 60% and specificity of 87% (95% CI: 0.718 to 0.819, p<0.001). The AUC of fasting times for solids was 0.753, with a cut-off value of 10.5, sensitivity of 62% and specificity of 86% (95% CI: 0.699 to 0.807, p<0.001). The AUC of fasting times for fluids was 0.768, with a cut-off value of 8.5, sensitivity of 64% and specificity of 74% (95% CI: 0.719 to 0.816, p<0.001).
Figure 3.
Risk factors for EA using the ROC curve. Predictive values of risk factors were assessed using the ROC curve. The VAS score for postoperative pain (AUC=0.769, 95% CI: 0.718 to 0.819, p<0.001), fasting times for solids (AUC=0.753, 95% CI: 0.699 to 0.807, p<0.001) and fasting times for fluids (AUC=0.768, 95% CI: 0.719 to 0.816, p<0.001) demonstrated good predictive effects. AUC, area under the ROC curve; EA, emergence agitation; ROC, receiver operating characteristic; VAS, Visual Analogue Scale.
Discussion
The results of this study indicated that EA was a common postoperative complication in patients who underwent general anaesthesia for TJA. Furthermore, this study identified four risk factors associated with EA in elderly patients who underwent TJA, including postoperative pain, CRBD, male sex and preoperative fasting times.
The incidence of EA was 37.6% in elderly patients who underwent TJA. To our knowledge, this report is the first on the incidence of EA in elderly patients who have undergone TJA. Previous research has shown that the incidence of EA varies widely. A prospective study demonstrated that 13.9% (158 of 1136) of adult patients had EA in the PACU.19 Mei and Tong7 reported that the incidence of EA in elderly patients who underwent gastrointestinal surgery was 40%. Moreover, an extremely high proportion of patients, 90.5% (19 of 21), experienced EA because of the effects of succinylcholine.20 These large differences may be attributed to the types of surgery, anaesthetic management, patient characteristics and assessment methods.
Many scales are available to assess EA in adults, including the RASS, Ricker Sedation-Agitation Scale, Aono’s 4-point scale and so on. Unlike the excellent reliability and validity in assessing sedation and agitation in the ICU,18 the reliability and validity of the RASS in the PACU have not been validated. Nevertheless, the RASS is easy to use and administer and has discrete criteria.18 Thus, we believe that RASS is an effective and efficient method of assessing EA in the PACU. Similarly, Makarem et al19 and Mei and Tong7 also chose the RASS to assess EA in the PACU.
Almost all researchers agree that postoperative pain is an independent risk factor for EA. Pain, an uncomfortable emotional experience, can lead to some complex neurobehavioural effects, such as agitation.21 Our study demonstrated that the VAS scores of patients in the EA group were higher than those in the non-EA group, and a postoperative pain VAS score ≥4 was the cut-off point for EA. Pain after TJA is common, and several studies have discovered that more than 50% of patients have suboptimal pain management after THA, and 75% of patients undergoing TKA reported moderate-to-severe pain.12 22 In this study, 72% (295 of 410) of patients reported pain, and 5% (21 of 410) of patients experienced severe pain, comparable with the results of previous reports. Yu et al23 found that nearly half of patients had EA because of insufficient postoperative analgesia. Peripheral nerve blocks (PNBs) can provide excellent analgesia.24 In our study, FNB was routinely used in patients undergoing TKA, and FICB was used for THA to improve postoperative analgesia. However, due to anatomical variations and individual characteristics, PNBs may not absolutely eliminate pain in patients undergoing TJA, leading to some patients experiencing EA due to postoperative pain in the study. Moreover, sore throat and catheter-related pain should not be ignored because postoperative pain is not limited to wound pain. Based on these findings, we strongly suggest that multimodal analgesia should be performed to benefit patients, especially with preventive analgesia.
The placement of an indwelling catheter is a common clinical procedure in the perioperative period. The collected urine is used for urine measurements and blood volume evaluation. However, patients with indwelling catheters are prone to CRBD.25 CRBD is characterised by discomfort confined to the suprapubic region, burning sensation, pain, and urinary urgency and frequency.26 27 CRBD can occur in 47%–90% of patients with an indwelling catheter,5 and CRBD can increase the incidence of EA and pain sensation after surgery.28 A retrospective study reported that approximately 10% of patients experienced EA during urological surgery, possibly related to CRBD.16 In our study, 28.0% (119 of 410) of patients experienced EA due to CRBD, and the higher incidence of EA may be due to the age of the recruited patients. This is because age ≥50 years was an independent predictor of CRBD.29 Indwelling catheters as a risk factor for EA have been reported previously in the literature.30 Early removal of indwelling catheters is helpful in decreasing EA associated with CRBD.
Regarding the effect of sex on EA, the results of the study are similar to those of reported in other studies in which male sex was identified as an independent risk factor for EA.29 This observation could be explained by several factors. First, male patients were high-risk patients with CRBD.29 Half of all men aged ≥50 years and over 80% of men aged ≥80 years have prostatic hyperplasia, which can easily cause discomfort and pain when the catheter tip contacts the bladder triangle on the pubis.31 Thus, male patients especially have difficulty tolerating the discomfort associated with catheters during the awakening period of anaesthesia. Furthermore, male patients have low postoperative pain tolerance, requiring more analgesics than female patients.32
Preoperative fasting is one of the preoperative instructions for patients. Whether preoperative fasting is a risk factor for EA has not been reported in previous studies. Prolonged preoperative fasting can cause metabolic, physical and psychological discomfort in patients, eventually leading to abnormal neurobehavioural changes, such as postoperative delirium.33 However, EA was not analysed. In this study, the fasting times of the EA group were significantly longer than those of the non-EA group and exceeded conventional fasting times (no more than 8 hours for solids and no more than 6 hours for liquids before surgery).34 Furthermore, 10.5 hours (fasting times for solids) and 8.6 hours (fasting times for fluids) are cut-off points for EA. Prolonged preoperative fasting times led to patient anxiety, and the degree of anxiety was related to the length of fasting time,34 while preoperative anxiety has been reported as a risk factor for EA.16 Due to the numerous patients and the lack of medical resources, patients may often experience longer fasting times than they were advised. To reduce the incidence of EA, effective preoperative education and scientific operation schedule lists should be developed.
This study had some limitations. First, we only included elderly patients who had undergone intravenous anaesthesia. Future studies may use other methods and anaesthetics. Second, this was a single-centre study; therefore, the generalisability of the results was not fully verified. Future multicentre studies must assess external validity. Lastly, this is a retrospective cohort study; thus, some bias is unavoidable. Future prospective cohort studies should evaluate and validate the risk factors for EA identified by our study.
Conclusions
In short, this retrospective study showed that EA is a common complication in elderly patients after TJA. EA occurred in 37.6% of the elderly patients who underwent TJA. Postoperative pain, CRBD, male sex and preoperative fasting times were independent predictors of EA. These risk factors may contribute to identifying high-risk patients to develop effective strategies to prevent and treat EA. Agitation has many causes35; therefore, the optimal clinical strategies should be multimodal.
Supplementary Material
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Contributors: ZL and JH have made substantial contributions to the conception or design of the manuscript. NW wrote this manuscript and made some changes after review. Furthermore, he worked with JD and JZ to acquire, analyse and interpret the data. All authors have participated in drafting the manuscript, and ZL revised it critically. All authors contributed equally to the manuscript and read and approved the final version of the manuscript. ZL is responsible for the overall content as a guarantor.
Funding: This work was supported by the Key Research and Development Program of Shaanxi, China (2019SF-205, 2022SF-149).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and was approved by the Biomedical Research Ethics Committee of Honghui Hospital (approval no. 201812001). All methods were performed according to relevant guidelines and regulations. The study obtained consent to gather patients’ medical record information through telephone follow-up.
References
- 1.Yang X, Hu Z, Peng F, et al. Effects of dexmedetomidine on emergence agitation and recovery quality among children undergoing surgery under general anesthesia: a meta-analysis of randomized controlled trials. Front Pediatr 2020;8:580226. 10.3389/fped.2020.580226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y, Zhang X, Sun Y, et al. Emergence agitation after the cleft lip or palate surgery in pediatric patients: a prospective study. J Stomatol Oral Maxillofac Surg 2021;122:539–43. 10.1016/j.jormas.2020.11.006 [DOI] [PubMed] [Google Scholar]
- 3.Kim H-J, Kim D-K, Kim H-Y, et al. Risk factors of emergence agitation in adults undergoing general anesthesia for nasal surgery. Clin Exp Otorhinolaryngol 2015;8:46. 10.3342/ceo.2015.8.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Z, Shi X, Yan X, et al. Comparing the effects of dexmedetomidine versus propofol on the treatment of emergence agitation in adult patients after general anesthesia: study protocol for a randomized, superiority, controlled trial (DP-TEA trial). Trials 2021;22:811. 10.1186/s13063-021-05743-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H, Pan Y, Liu C, et al. Emergence agitation after intraoperative neurolytic celiac plexus block with alcohol: a case report. BMC Anesthesiol 2021;21:204. 10.1186/s12871-021-01426-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol 2020;73:471–85. 10.4097/kja.20097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei X, Tong J. The plasma levels of brain-derived neurotrophic factor are positively associated with emergence agitation in the elderly after gastrointestinal surgery. J Anesth 2016;30:811–6. 10.1007/s00540-016-2212-3 [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Peng T, Sun Y, et al. Intraoperative use of low-dose dexmedetomidine for the prevention of emergence agitation following general anaesthesia in elderly patients: a randomized controlled trial. Aging Clin Exp Res 2022;34:611–8. 10.1007/s40520-021-01984-y [DOI] [PubMed] [Google Scholar]
- 9.Niu R, Egan C, Fang C, et al. Total joint arthroplasty in homeless patients at an urban safety net Hospital. J Am Acad Orthop Surg 2022;30:523–7. 10.5435/JAAOS-D-21-00651 [DOI] [PubMed] [Google Scholar]
- 10.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 2021;325:568–78. 10.1001/jama.2020.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Zhou X, Zhang Y, et al. Predictors of postoperative delirium in elderly patients following total hip and knee arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord 2021;22:945. 10.1186/s12891-021-04825-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Luca ML, Ciccarello M, Martorana M, et al. Pain monitoring and management in a rehabilitation setting after total joint replacement. Medicine (Baltimore) 2018;97:e12484. 10.1097/MD.0000000000012484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei B, Feng Y, Chen W, et al. Risk factors for emergence agitation in adults after general anesthesia: a systematic review and meta-analysis. Acta Anaesthesiol Scand 2021;65:719–29. 10.1111/aas.13774 [DOI] [PubMed] [Google Scholar]
- 14.Demir CY, Yuzkat N. Prevention of emergence agitation with ketamine in rhinoplasty. Aesth Plast Surg 2018;42:847–53. 10.1007/s00266-018-1103-4 [DOI] [PubMed] [Google Scholar]
- 15.Tolly B, Waly A, Peterson G, et al. Adult emergence agitation: a veteran-focused narrative review. Anesth Analg 2021;132:353–64. 10.1213/ANE.0000000000005211 [DOI] [PubMed] [Google Scholar]
- 16.Kim H-C, Kim E, Jeon Y-T, et al. Postanaesthetic emergence agitation in adult patients after general anaesthesia for urological surgery. J Int Med Res 2015;43:226–35. 10.1177/0300060514562489 [DOI] [PubMed] [Google Scholar]
- 17.Kang X, Lin K, Tang H, et al. Risk factors for emergence agitation in adults undergoing thoracoscopic lung surgery: a case-control study of 1,950 patients. J Cardiothorac Vasc Anesth 2020;34:2403–9. 10.1053/j.jvca.2020.02.046 [DOI] [PubMed] [Google Scholar]
- 18.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–44. 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 19.Makarem J, Larijani AH, Eslami B, et al. Risk factors of inadequate emergence following general anesthesia with an emphasis on patients with substance dependence history. Korean J Anesthesiol 2020;73:302–10. 10.4097/kja.19214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S-J, Sung T-Y, Cho C-K. Comparison of emergence agitation between succinylcholine and rocuronium-sugammadex in adults following closed reduction of a nasal bone fracture: a prospective randomized controlled trial. BMC Anesthesiol 2019;19:228. 10.1186/s12871-019-0907-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menser C, Smith H. Emergence agitation and delirium: considerations for epidemiology and routine monitoring in pediatric patients. Local Reg Anesth 2020;13:73–83. 10.2147/LRA.S181459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlenwein J, Müller M, Falla D, et al. Clinical relevance of persistent postoperative pain after total hip replacement-a prospective observational cohort study. J Pain Res 2017;10:2183–93. 10.2147/JPR.S137892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu D, Chai W, Sun X, et al. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anaesth 2010;57:843–8. 10.1007/s12630-010-9338-9 [DOI] [PubMed] [Google Scholar]
- 24.Dotto A, Dunsmuir D, Sun T, et al. The use of the panda-nerve block pain APP in single-shot peripheral nerve block patients: a feasibility study. Can J Anaesth 2020;67:1140–51. 10.1007/s12630-020-01732-2 [DOI] [PubMed] [Google Scholar]
- 25.Jang EB, Hong SH, Kim KS, et al. Catheter-Related bladder discomfort: how can we manage it? Int Neurourol J 2020;24:324–31. 10.5213/inj.2040108.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach H, Kaasby K, Sørensen A, et al. Incidence and severity of catheter-related bladder discomfort among nonurological adult patients in a postanesthesia care unit. J Perianesth Nurs 2020;35:29–33. 10.1016/j.jopan.2019.06.013 [DOI] [PubMed] [Google Scholar]
- 27.Kim D-H, Park J-Y, Yu J, et al. Intravenous lidocaine for the prevention of postoperative catheter-related bladder discomfort in male patients undergoing transurethral resection of bladder tumors: a randomized, double-blind, controlled trial. Anesth Analg 2020;131:220–7. 10.1213/ANE.0000000000004405 [DOI] [PubMed] [Google Scholar]
- 28.Binhas M, Motamed C, Hawajri N, et al. Predictors of catheter-related bladder discomfort in the post-anaesthesia care unit. Annales Françaises d’Anesthésie et de Réanimation 2011;30:122–5. 10.1016/j.annfar.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 29.Li SY, Song LP, Ma YS, et al. Predictors of catheter-related bladder discomfort after gynaecological surgery. BMC Anesthesiol 2020;20:97. 10.1186/s12871-020-01018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fields A, Huang J, Schroeder D, et al. Agitation in adults in the post-anaesthesia care unit after general anaesthesia. Br J Anaesth 2018;121:1052–8. 10.1016/j.bja.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 31.Chughtai B, Forde JC, Thomas DDM, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers 2016;2:16031. 10.1038/nrdp.2016.31 [DOI] [PubMed] [Google Scholar]
- 32.Tsui SL, Tong WN, Irwin M, et al. The efficacy, applicability and side-effects of postoperative intravenous patient-controlled morphine analgesia: an audit of 1233 Chinese patients. Anaesth Intensive Care 1996;24:658–64. 10.1177/0310057X9602400604 [DOI] [PubMed] [Google Scholar]
- 33.Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth 2020;125:492–504. 10.1016/j.bja.2020.06.063 [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Lu Q, Wang B, et al. Preoperative fasting times for patients undergoing elective surgery at a pediatric hospital in Shanghai: the big evidence-practice gap. J Perianesth Nurs 2021;36:559–63. 10.1016/j.jopan.2021.01.005 [DOI] [PubMed] [Google Scholar]
- 35.Yan L-M, Chen H, Yu R-G, et al. Emergence agitation during recovery from intracranial surgery under general anaesthesia: a protocol and statistical analysis plan for a prospective multicentre cohort study. BMJ Open 2015;5:e007542. 10.1136/bmjopen-2014-007542 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available.