FIGURE 7.
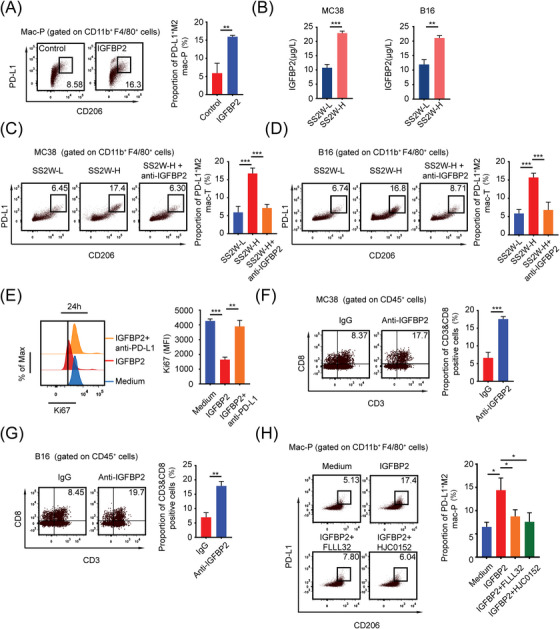
HTB induces PD‐L1+ M2‐like macrophages through IGFBP2‐STAT3 axis, (A) Mac‐Ps were stimulated with or without IGFBP2 (10 ng/mL) for 24 h. PD‐L1+ M2 mac‐Ps were detected by flow cytometry (n = 3), (B) ELISA detected IGFBP2 content between SS2W‐L and SS2W‐H MC38 or B16 subcutaneous tumor conditioned medium (n = 3), (C) Flow cytometry revealed that IGFBP2‐neutralizing antibody (anti‐IGFBP2, 10 mg/kg, intraperitoneal injection on day 7) blocked M2‐like polarization of intratumoral macrophages in HTB MC38 subcutaneous tumors in vivo (n = 3), (D) Flow cytometry revealed that IGFBP2‐neutralizing antibody (10 mg/kg, intraperitoneal injection on day 7) blocked M2‐like polarization of intratumoral macrophages in HTB B16 subcutaneous tumors in vivo (n = 3), (E) Mac‐Ps were preactivated by IGFBP2 for 24 h, co‐cultured with CD8+ T cells with or without anti‐PD‐L1 antibody (5 μg/mL). Flow cytometry revealed that IGFBP2‐induced mac‐Ps inhibit CD8+ T cells through PD‐1/PD‐L1 (n = 3), (F) Flow cytometry revealed that anti‐IGFBP2 injection at an early tumor age (day 7) promoted the infiltration of intratumoral CD8+ T cells when MC38 subcutaneous tumors grew into HTB (n = 3), (G) Flow cytometry revealed that anti‐IGFBP2 injection at an early tumor age (day 7) promoted the infiltration of intratumoral CD8+ T cells when B16 subcutaneous tumors grew into HTB (n = 3), (H) Flow cytometry revealed that IGFBP2 induces PD‐L1+ M2 mac‐Ps through STAT3. Mac‐Ps were stimulated with complete medium or IGFBP2 (10 ng/mL) or IGFBP2 & FLLL32 (5 μmol/L) or IGFBP2 & HJC0152 (5 μmol/L) for 24 hours. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Abbreviations: LTB, low tumor burden; HTB, high tumor burden; mac‐P, peritoneal macrophage; mac‐T, tumor‐infiltrating macrophage; PD‐L1, programmed death‐ligand 1; IGFBP2, insulin‐like growth factor binding protein 2; PD‐1, programmed cell death 1; STAT3, signal transducer and activator of transcription 3.
