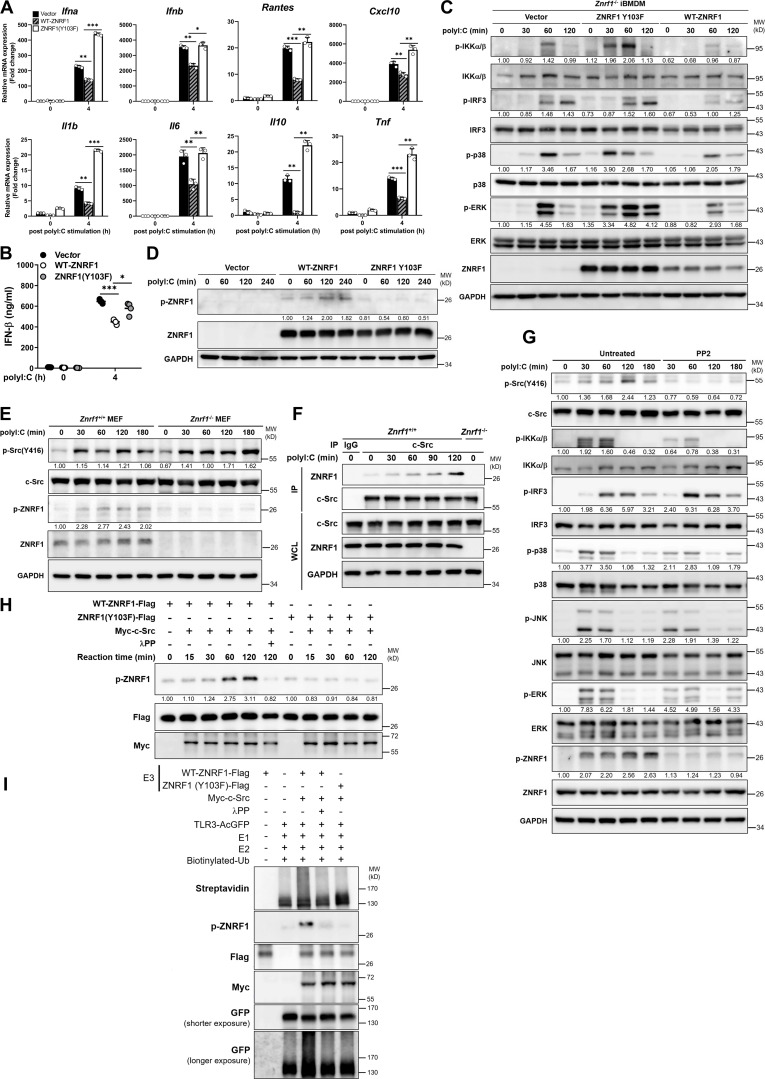
Figure 8.
ZNRF1-mediated TLR3 polyubiquitination requires activation by c-Src via phosphorylation at its Y103. (A–C) Znrf1−/− iBMDMs were reconstituted with Tet-inducible vector, wild-type ZNRF1, or ZNRF1(Y103F) mutant, and stimulated with poly(I:C) (30 μg/ml) for the times indicated. (A) The expression of the mRNAs indicated was analyzed by RT-qPCR. (B) The secreted IFN-β in culture media after stimulation with poly(I:C) for 4 h was measured by ELISA analysis. (C) Cell lysates were subjected to immunoblotting with the antibodies indicated. (D) Znrf1−/− RAW264.7 reconstituted with vector, wild-type ZNRF1, or ZNRF1(Y103F) mutant were stimulated with poly(I:C) (30 μg/ml) for the times indicated. Cell lysates were analyzed by immunoblotting. (E) Znrf1+/+ and Znrf1−/− MEFs were stimulated with poly(I:C) (100 μg/ml) for the times indicated and cell lysates were analyzed by immunoblotting. The intensities of the bands are expressed as fold increases compared with those of untreated control cells after normalization to their unphosphorylated forms. (F) Znrf1+/+ or Znrf1−/− BMDMs were stimulated with poly(I:C) (30 μg/ml) for the times indicated. Cell lysates were immunoprecipitated with either IgG or anti–c-Src antibody, and the immunocomplexes and WCL were subjected to immunoblotting with the antibodies indicated. (G) BMDMs from Znrf1+/+ mice were either pretreated with or without PP2 (10 μM) for 1 h, followed by stimulation with poly(I:C) (30 μg/ml) in the absence of PP2 for the times indicated. Cell lysates were collected and analyzed by immunoblotting with the antibodies indicated. (H) HEK293T was transfected with Flag-tagged wild-type ZNRF1, ZNRF1(Y103F), or Myc-tagged c-Src for 24 h. Cell lysates were immunoprecipitated with anti-M2-Flag or anti-Myc antibodies. In vitro kinase assays were performed with immunoprecipitated Flag-tagged wild-type ZNRF1 or ZNRF1(Y103F) and Myc-tagged c-Src with or without λPP as indicated at 30°C for the reaction times indicated, followed by immunoblotting with the antibodies indicated. The intensities of the bands are expressed as fold increases compared with those of untreated control cells, after normalization to their unphosphorylated forms. (I) In vitro ubiquitination assays were carried out with Flag-tagged wild-type ZNRF1 or ZNRF1(Y103F) incubated with Myc–c-Src prepared from H and AcGFP-tagged TLR3 immunopurified from HEK293T cells transfected with AcGFP-TLR3 and recombinant ubiquitin catalytic components as indicated at 37°C for 3 h. The mixtures were then subjected to immunoblotting with the antibodies indicated. *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test). Data are representative of three independent experiments (error bars, mean ± SD). Source data are available for this figure: SourceData F8.