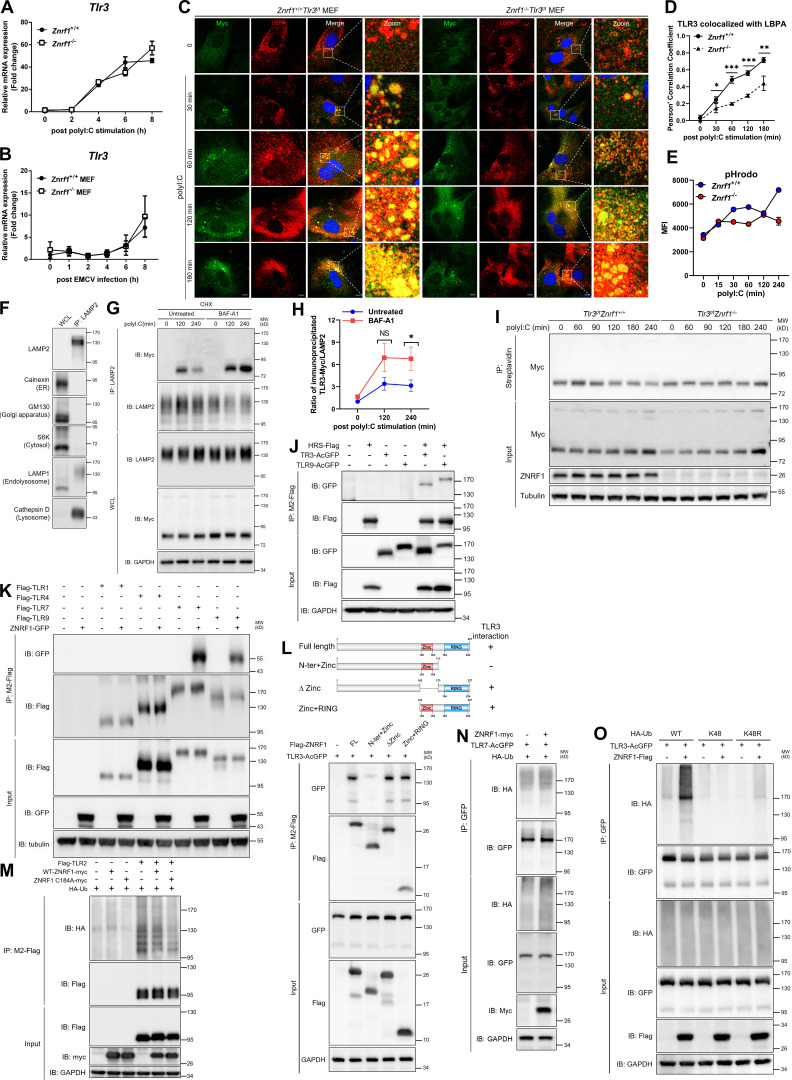
Figure S4.
ZNRF1 does not affect TLR3 mRNA expression upon ligand binding and does not mediate K48-linked polyubiquitin chains on TLR3. (A and B) RT-qPCR analysis of the mRNA expression of Tlr3 in BMDMs and MEFs from Znrf1+/+ and Znrf1−/− mice after stimulation with poly(I:C) (30 μg/ml) or EMCV at an MOI of 1 for the times indicated. (C and D) Znrf1+/+Tlr3t/t and Znrf1−/−Tlr3t/t MEFs were not treated or treated with poly(I:C) (100 μg/ml) for the times indicated. (C) Cells were costained with antibodies against Myc (TLR3) and LBPA. Scale bar, 10 μm. (D) Quantitative analysis of colocalization of TLR3 with LBPA. (E) Znrf1+/+ and Znrf1−/− BMDMs were stimulated with poly(I:C) (30 μg/ml) for the times indicated. Cells were incubated with pHrodo green for 15 min followed by flow cytometric analysis. MFI, mean fluorescence intensity. (F) Cell lysates were prepared from BMDMs and immunoprecipitated with LAMP2 antibodies. (G and H) WCL and purified LAMP2+ vesicles were subjected to immunoblotting using the antibodies against proteins of various subcellular compartments (Calnexin: ER, GM130: Golgi apparatus, S6K: cytosol, LAMP1: endolysosomes/lysosomes, and Cathepsin D: lysosomes; G and H) Znrf1+/+Tlr3t/t BMDMs were pretreated with CHX (10 μg/ml) for 1 h and then stimulated with poly(I:C) (30 μg/ml) for 30 min, followed by treatment with Bafilomycin A1 (BAF-A1; 2 μM) for the times indicated. Cell lysates were prepared and immunoprecipitated with LAMP2 antibodies followed by immunoblotting (IB) with the antibodies indicated (G). The intensities of the immunoprecipitated Myc bands are expressed as fold increases compared to those of untreated control cells, after normalization to their immunoprecipitated LAMP2 (H). (I) Znrf1+/+Tlr3t/t and Znrf1−/−Tlr3t/t BMDMs were methionine-starved for 1 h, and then fed with L-Azidohomoalanine for 4 h, followed by poly(I:C) (30 μg/ml) stimulation for the times indicated. Cell lysates were crosslinked with Biotin-alkyne and then immunoprecipitated with anti-Streptavidin antibody, followed by immunoblotting with the antibodies indicated. (J) HEK293T were co-transfected with HRS-Flag, TLR3-AcGFP, or TLR9-AcGFP for 72 h, and cell lysates were immunoprecipitated with anti-M2-Flag antibody. Immunocomplexes and WCL were subjected to immunoblotting with the antibodies indicated. (K) HEK293T were cotransfected with ZNRF1-GFP or Flag-tagged TLR1 or TLR4 or TLR7 or TLR9 for 36 h, and cell lysates were immunoprecipitated with anti-M2-Flag antibody. Immunocomplexes and WCL were subjected to immunoblotting with the antibodies indicated. (L) HEK293T cells were cotransfected with AcGFP-tagged TLR3 and Flag-tagged full-length (FL) or truncated forms of ZNRF1 for 72 h, and the interaction between TLR3 and ZNRF1 was identified by immunoprecipitation followed by immunoblotting with the antibodies indicated. Schematic diagram of full-length ZNRF1 and its various deletion mutants, with a C-terminal Flag tag, is shown in the upper panel. (M) HEK293T cells were cotransfected with Flag-tagged TLR2, HA-tagged ubiquitin, and myc-tagged wild-type ZNRF1 or ZNRF1(C184A) for 36 h. (N) HEK293T cells were cotransfected with TLR7-AcGFP, ZNRF1-myc, and HA-tagged ubiquitin for 36 h. (O) HEK293T cells were cotransfected with TLR3-AcGFP, ZNRF1-Flag, and HA-tagged wild-type or ubiquitin mutants for 36 h. Cell lysates were immunoprecipitated using anti-GFP antibody. The immunocomplexes and WCL were analyzed by immunoblotting using the antibodies indicated. Data are representative of two independent experiments (error bars, mean ± SD). Source data are available for this figure: SourceData FS4.