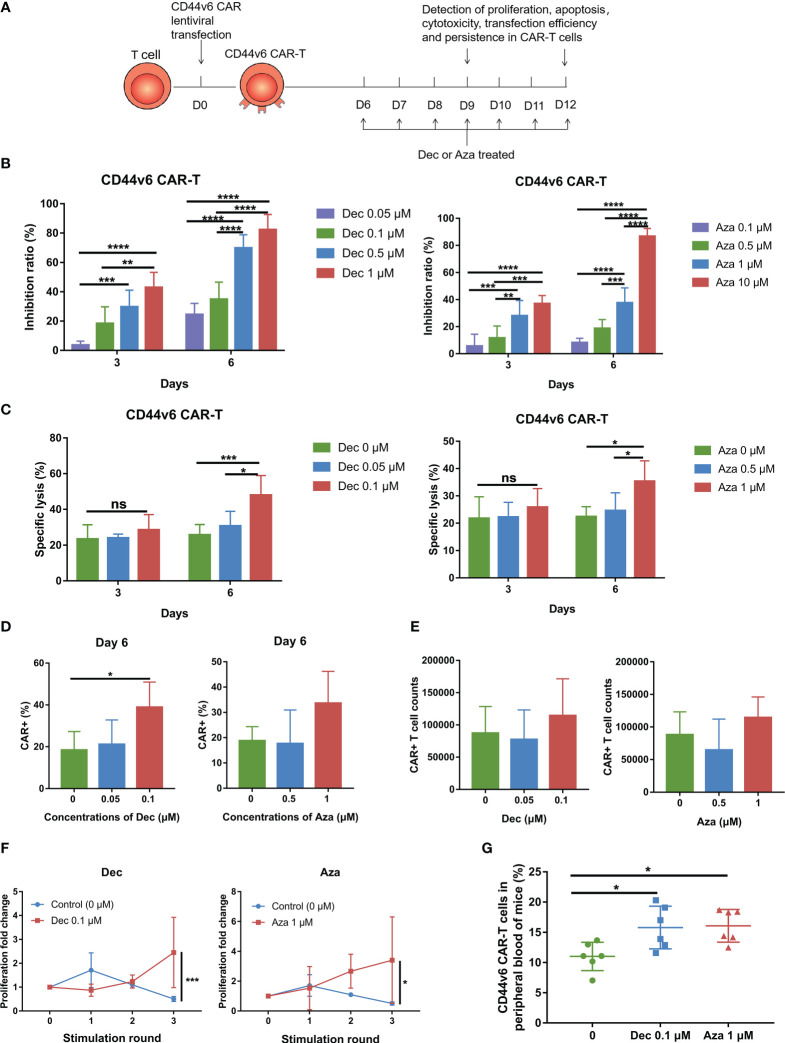
Figure 1.
Dec and Aza treated CD44v6 CAR-T cells exhibit increased CAR+ cells and persistence. (A) Experimental design of CD44v6 CAR-T cells treated with Dec and Aza. (B) Inhibition ratio of CD44v6 CAR-T cells treated with various concentrations of Dec (left, n=6) and Aza (right, n=8) for 3 or 6 days. (C) Cytotoxicity of CD44v6 CAR-T cells treated with Dec (0.05, 0.1 μM) (left, n=6) and Aza (0.5, 1 μM) (right, n=5) for 3 or 6 days against MV4-11 cells in an E:T ratio of 10:1 for 24 h. (D, E) Percentage (D) and absolute number (E) of CAR+ cells in CD44v6 CAR-T cells treated with Dec (0.05, 0.1 μM) (n=4) and Aza (0.5, 1 μM) (n=5) for 6 days. (F) Proliferation fold of CD44v6 CAR-T cells treated with Dec 0.1uM and Aza 1uM for 6 days in response to repeated stimulation of MV4-11 cells (n=4). (G) CD44v6 CAR-T cells treated with or without Dec or Aza were injected intravenously into NCG mice simultaneously with MV4-11, and the percentage of CD44v6 CAR-T cells in peripheral blood was detected by flow cytometry 3 weeks later (n=6). CD44v6, CD44 isoform variant 6; CAR-T, chimeric antigen receptor T; Dec, decitabine; Aza, azacitidine. Data are depicted as the mean± SD. *p<.05; **p<.01; ***p<.001; ****p<.0001; ns, not significant.