Abstract
Background and aims.
Although the co-occurrence of cannabis and depression is well-established, less is known about the temporal sequence of cannabis use and depression. The present study had three main aims: to test a symptom-driven pathway in which depression may drive increases in cannabis use, to test a substance-induced pathway in which cannabis use may drive increases in depression and to assess a shared vulnerability model assessing associations between individuals who have (and have not) experienced adverse childhood experiences (ACEs).
Design.
Data are from an ongoing, longitudinal, cohort study (n = 2,234). Data were set up in accelerated longitudinal design from 17 to 24 years old.
Setting.
Initial sample was recruited from Southern California, USA. The majority of participants still live in Southern California.
Participants.
On average, participants were 18 years old at wave 8 with over half identifying as female (54.3%; n = 1,350). Most participants identified as Hispanic (1,127; 45.4%), followed by non-Hispanic White (510; 20.5%), Asian (503; 20.2%), Multiracial/Other (284; 11.4%), and non-Hispanic Black (60; 2.2%).
Measurements.
Primary outcomes were past month days of cannabis use and depression symptoms (PHQ-8). The Adverse Childhood Experiences Scale was used as our main grouping measure.
Findings.
In the full sample we showed that prior levels of depression symptoms were associated with a decrease in cannabis use (opposite of the proposed symptom driven model; B = −0.33 [−0.58, −0.09]). Dynamic coupling parameters noted individuals who evidenced greater increases in cannabis use between two prior ages reported greater increases in depressive symptoms between subsequent ages (support for a substance induced pathway; B = 0.53 [0.18, 0.89]). Similar to the overall sample, for those who had not experienced ACEs, as cannabis use increased we saw a steady increase in depression (support for a substance induced pathway; B = 0.14 [0.04, 0.29]). However, for those who experienced ACEs, as cannabis use increased we saw a consistent decrease in depression (opposite of the proposed substance-induced pathway; B= −0.18 [−0.28, −0.08]).
Conclusion.
There is mixed support for both symptom driven and substance-induced pathways between cannabis use and depression.
INTRODUCTION
Cannabis is one of the most widely used substances among young adults (ages 18 to 25) in the United States, with 34.5% (11.6 million) reporting past year use – a prevalence rate that has remained relatively stable since 2015 (1). The changing landscape of cannabis in the U.S. raises important concerns about how young people may be adversely affected, particularly with respect to mental health. Depression is one such mental health disorder linked to cannabis use in young adulthood (2,3), and an important correlate of both cannabis use and depression is exposure to adverse childhood experiences (ACEs: sexual abuse, physical abuse, neglect, and emotional abuse). Numerous studies have noted greater risk of long-term cannabis use and depressive episodes among those who have experienced ACEs versus those who have not (4). The present study is designed to provide more clarity on the complex and dynamic interplay between cannabis use and depression from late adolescence through young adulthood and how these associations may vary by ACEs.
Although there is a relatively robust literature noting an association between cannabis use and the development of psychotic disorders (5), there is less consensus about the directionality between cannabis use and depression (2,3). There are two common explanations for the association between cannabis use and depression. The first is that prolonged use of cannabis can prompt, or exacerbate, severity of depressive symptomology-- a substance-induced pathway. Large representative cross-sectional studies from the United States, Australia, and New Zealand (6) have provided support for this pathway, although others have failed to show an association or noted greater cannabis use is associated with less depressive symptomology (7). The second explanation is that individuals use substances (cannabis) to self-regulate and mitigate mental health symptomology (depression)--a symptom-driven pathway (sometimes referred to as the self-medication hypothesis), in which depressive symptomology precedes greater cannabis use. Again, some prior cross-sectional research has supported this hypothesis, whereas other studies have noted no association (3).
Cross-sectional support is informative; however, it does not allow for assessment of directionality. An early review of research assessing associations between depression and cannabis use concluded that, for the most part, longitudinal studies do not support the symptom-driven pathway (i.e., depressive symptoms predicting later cannabis use) among young adults (6). A decade later, Lev-Ran and colleagues (2014) meta-analyzed 57 longitudinal studies assessing which patterns of cannabis use were associated with development of depression (substance-induced pathway). In general, those using cannabis (versus non-use) and those using heavily (versus light or non-use) evidenced greater odds of developing depression later in life. Most recently, Garey and colleagues (2020) reviewed longitudinal, prospective studies on directional associations between depression and cannabis use. They concluded that current literature presents mixed evidence for both symptom-driven and substance-induced pathways. Thus, nearly 20 years after the initial review (6), evidence is even less clear on directionality between depression and cannabis use. In fact, if we look at recent studies testing the symptom-driven pathway, results indicate that a diagnosis of major depressive disorder during young adulthood is associated with reduced frequency of cannabis use in adulthood, contrary to the symptom-driven pathway, whereas others find positive associations between depressive symptoms in adolescence and greater likelihood of developing cannabis use disorder in young adulthood (8).
There are reasons for lack of consensus, including methodological limitations and lack of focus on shared vulnerability models. Regarding methods, most longitudinal studies use summary data from one developmental period, such as cannabis use during adolescence, to predict an outcome at another developmental period, such as depressive symptoms in young adulthood. Prior research has also utilized methods such as cross-lagged panel models to assess time-lagged associations between cannabis and depression, with mixed results (9,10). Unfortunately, cross-lagged panel models provide estimates that are an amalgam of within- and between-person variance and do not account for change, which make results difficult to interpret (11,12). Many studies also fail to address important shared vulnerabilities. In their conclusion, Garey and colleagues (2020) note that, although evidence is mixed on the longitudinal association between cannabis use and depression, there is evidence for a shared vulnerability model in which this association varies by important demographic factors. One common, and important, correlate of both depression and cannabis use is exposure to ACEs. There is robust literature linking ACEs to depressive disorders and cannabis use later in life (13-15); however, few studies have assessed effects of ACEs on the dynamic interplay between depression and cannabis use.
The Present Study
The present study extends our current understanding of directional associations between cannabis use and depression from late adolescence through young adulthood (17 – 24 years old). First, we examined whether depression was associated with greater cannabis use throughout young adulthood (Aim 1- symptom-driven pathway). Second, we assessed whether cannabis use was associated with greater depression symptoms throughout young adulthood (Aim 2-substance-induced pathway). Third, we tested a shared vulnerability model by examining whether ACEs accounts for differential associations between cannabis use and depressive symptoms from late adolescence through young adulthood (Aim 3). Given the lack of consensus in the literature and the complex methodology used in the present study results should be considered exploratory and used as a starting point for more detailed understanding of the associations between cannabis use and depression.
METHODS
Procedures
Participants are from two cohorts of students in 6th and 7th grade recruited in 2008 from 16 middle schools in Southern California for an evaluation of a voluntary after-school substance use prevention program, CHOICE (16). Study procedures are reported in full elsewhere (17). Briefly, individuals were followed across 13 annual survey waves through 2020; those who do not complete the survey in a given wave remain eligible to complete subsequent surveys. Retention averages 85% across all 13 waves, with 90.5% at wave 8, 89.1% at wave 9, 90.4% at wave 10, 92.3% at wave 11, 92.1% at wave 12, and 87.8% at wave 13 ( average 90% from waves 8-13). Wave-to-wave retention has never been associated with past month substance use (i.e., alcohol, cannabis, cigarettes), and retention was not significantly associated with participant demographics (e.g., age, sex) until wave 11. From wave 12 to wave 13, retention was not predicted by wave 12 substance use; however, retention was slightly higher among women than men (89.5% vs. 85.6%, respectively) and for those who were younger versus older (mean age = 22.5 vs. 22.9 at wave 12, respectively).
Participants
All prospective analyses used data from wave 8 when participants were between the ages of 17 and 20, through wave 13, when participants are between ages 21 and 24. On average, participants were 18 years old at wave 8 with over half identifying as female (54.3%; n = 1,350). In terms of cannabis use, 23.8% (n = 590) reported past month cannabis use with an average, of 2.3 days and 5.8% (n = 143) reported daily cannabis use. Participants also reported 4.8 depressive symptoms in the past month with 12.4% of participants meeting the cutoff (10 symptoms) for probable depressive disorder. Regarding ACEs, 28% (n = 700) reported at least one adverse childhood event, and 18% reported two or more adverse events (see Table 1).
Table 1.
Participant demographic characteristics and behavioral health from wave 8
| Total Sample N = 2,234 |
ACEs+ N = 700 |
ACEs− N = 1,534 |
|
|---|---|---|---|
| Variable | M(SD) or n(%) |
M(SD) or n(%) |
M(SD) or n(%) |
| Age | 18.3 (0.73) | 18.3 (0.74) | 18.3 (0.72) |
| Female | 1,350 (54.3%) | 425 (60.7%) | 835 (52.7%) |
| Race/Ethnicity | |||
| Non-Hispanic White | 510 (20.5%) | 129 (18.4%) | 345 (21.8%) |
| Non-Hispanic Black | 60 (2.24%) | 20 (2.9%) | 34 (2.1%) |
| Hispanic | 1,127 (45.4%) | 336 (48.0%) | 694 (43.8%) |
| Asian | 503 (20.2%) | 141 (20.1%) | 329 (20.8%) |
| Multiracial/Other | 284 (11.4%) | 74 (10.6%) | 182 (11.5%) |
| Mother’s Education | |||
| High school or less | 733 (29.5%) | 233 (33.3%) | 438 (27.6%) |
| Days of Cannabis Use (Past Month) | 2.34 (6.26) | 2.76 (6.72) | 2.07 (5.90) |
| Any past month cannabis use | 590 (23.8%) | 195 (27.9%) | 341 (21.5%) |
| Daily cannabis use | 143 (5.8%) | 47 (6.7%) | 79 (5.0%) |
| Depression | 4.12 (5.04) | 6.83 (6.06) | 3.95 (4.46) |
| Adverse Childhood Experiences | |||
| Humiliate or insult you | 376 (15.1%) | 376 (53.7%) | - |
| Felt no one loved you | 373 (15.0%) | 373 (53.3%) | - |
| Felt like someone in your family hated you | 388 (15.6%) | 388 (55.4%) | - |
| Physical abuse (hit, slapped, made marks) | 234 (9.4%) | 234 (33.4%) | - |
| Sexual abuse (touch, grabbed, attempt) | 174 (7.0%) | 174 (24.9%) | - |
| Witnessed parental violence (saw caregiver get hit, pushed, slapped) | 165 (6.8%) | 165 (23.6%) | - |
Measures
Background covariates.
Participants reported age, sex at birth (female vs. male), race/ethnicity [non-Hispanic white (reference), non-Hispanic black, Hispanic, Asian, and Other/Multiracial], and mother’s education (dichotomous indicator of family socioeconomic status; 1= “high school or less”). We controlled for past month alcohol and tobacco use frequency at wave 8 and CHOICE intervention status at wave 1 in 2008 (note that intervention effects on substance use were no longer significant after one year).
Cannabis use (past month).
Participants reported number of days they used cannabis in the past month (0 to 30 days) for waves 8 through 13.
Depression.
The PHQ-8 (reliability range: α = 0.94 [wave 8] to α = 0.95 [wave 13] ) assessed 8 symptoms of depression at waves 8 through 13, such as “little interest or pleasure in doing things” using the standard anchor of past two-week symptomology (scale from 0 = not at all to 3 = nearly every day). Scores were summed (total score range 0 – 21).
Adverse Childhood Experiences (ACEs).
Using items derived from the Adverse Childhood Experiences Scale (18), each participant answered questions regarding their experiences prior to their 18th birthday. Items covered emotional abuse, physical abuse, sexual abuse, and indirect violence. Each participant answered “yes” or “no” to each item. A dichotomous indicator was used as our grouping variable to indicate those who have and have not experienced ACEs.
Analytic plan
We used an accelerated longitudinal cohort design because data were set up in naturally occurring cohorts (e.g., age). This allows us to model developmental trajectories of depression symptoms and cannabis use from ages 17 to 24 years old.
Latent difference score model.
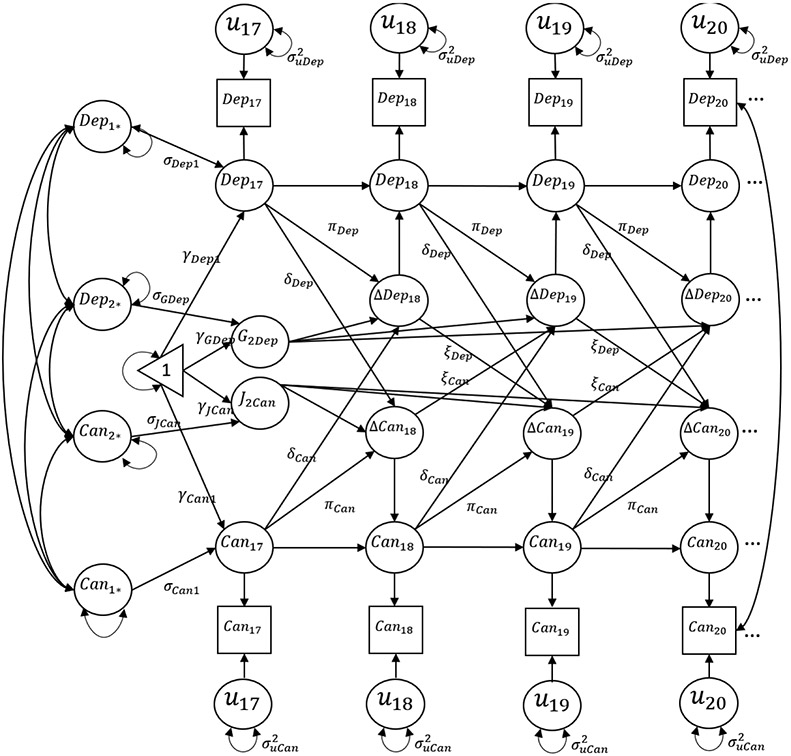
We used latent difference score models (19) to address study aims with the full sample (n = 2,284), and separate models for those who experienced ACEs (ACES+ ; n = 700) and those who had not experienced ACEs (ACES− ; n = 1,584). See Figure 1 for conceptual model. The latent difference score modeling approach allows for simultaneous examination of constant change (e.g., latent growth models), proportional change (how does previous level in one variable relate to subsequent change in another), and dynamic change (how does previous change in one variable relate to subsequent change in another). For this study, we focus on two within-person parameters. The first is the proportional coupling parameter, which models lagged effect of changes in, for example, cannabis use at a given age resulting from an individual’s level of depressive symptoms at the prior age. One would interpret a significant positive effect of this parameter as an individual’s level of depression at age t is associated with positive changes in (e.g., greater) cannabis use from age t to t+1.
Figure 1.
Conceptual latent difference score model for depression and cannabis use. Note: conceptual model stops at age 20 for visual purposes only. The full model extends to 24 years old.
The second component is the dynamic coupling parameter. Here, we examine how lagged change (rather than level) in one variable is associated with subsequent change in another variable. For example, it may be that changes in cannabis use are affected by specific levels of depression, but these changes in cannabis use are further accelerated when depression symptoms have recently increased. A significant positive effect would imply that increases in depression symptoms between two years in age (e.g., 18 and 19 years old) are associated with positive changes in cannabis use (e.g., greater use) between two subsequent time points for a given individual (e.g., 19 and 20 years old). As recommended by Grimm et al., (2016) we constrained each proportional change lag to be equal over time for parsimony and to allow for replication. All models were estimated using Mplus software, version 8.6 (20).
Visual representation of results.
In addition to discussing model parameters we also provide a visual representation of our results. In particular, we give examples of how depression changes for a hypothetical young adult when we force increases (shock) in cannabis use and vice versa. These results are used as a way to better visualize and understand the complex and dynamic associations between cannabis use and depression symptoms.
Missing data.
Prior to setting up data in an accelerated longitudinal design (N = 2,507) in order to have enough people in the ‘tails’ of our developmental cohort we removed 23 individuals (which is .009% of the sample) who were age 16 (n = 11), age 21 (n = 4), and age 22 (n = 8) years old at wave 8 (N = 2,484). Due to missing data on some exogenous variables, our final analytic sample size is 2,284. We used the robust maximum likelihood estimator, which can accommodate missing data on endogenous variables and non-normality and provide unbiased estimates.
RESULTS
For our final bivariate model, we describe the two within-person lagged coupling parameters. We provide full supplemental materials with methods, results, and discussion for each univariate model, the final bivariate model for the whole sample, as well as results for all between-person effects.
Within-Person Associations between Depression and Cannabis Use for Whole Sample
Table 2 provides final model estimates. Results from our proportional coupling parameters are in opposition to a symptom-driven pathway noting that greater prior levels of depression are associated with negative change in cannabis use (e.g., decreasing cannabis use; β = −0.33). That is, our partial effect is interpreted as a unit increase in depression at, for example age 18, is associated with a −.33 change in cannabis use from 18 to 19 years old. However, when assessing dynamic coupling parameters, a different story emerges. In particular, results support symptom-driven pathways such that positive prior changes in depression (increasing depression) are associated with positive changes in cannabis use (increasing cannabis use; β = 0.42). Results also support substance-induced pathways such that increasing cannabis use between 19 and 20 years old, for example, is associated with increasing depression between subsequent ages, 20 and 21 years old (β = 0.54).
Table 2.
Coupling parameters for the final bivariate latent difference sore model assessing longitudinal associations between depression symptoms and cannabis use
| Total Sample | ACEs+ | ACEs− | ||||
|---|---|---|---|---|---|---|
| Parameters for latent difference score model | B(SE) | p | B(SE) | p | B(SE) | p |
| Within construct proportional change | ||||||
| Level cannabis use→ changes cannabis use | −0.07 (0.03) | 0.01 | −0.28 (0.09) | <0.01 | 0.74 (0.26) | 0.01 |
| Level depression→ changes depression | −0.04 (0.08) | 0.59 | 0.13 (0.25) | 0.61 | 0.32 (0.09) | <0.01 |
| Proportional coupling parameters | ||||||
| Level cannabis use→ changes depression | 0.009 (0.02) | 0.70 | −0.18 (0.05) | <0.01 | −0.002 (0.01) | 0.93 |
| Level depression→ changes cannabis use | −0.33 (0.13) | 0.01 | 0.68 (0.21) | <0.01 | −1.33 (1.44) | 0.35 |
| Dynamic coupling parameters | ||||||
| Changes cannabis use→ changes depression | 0.54 (0.18) | <0.01 | −0.31 (0.33) | 0.35 | 0.14 (0.07) | 0.04 |
| Changes depression→ changes cannabis use | 0.42 (0.21) | 0.04 | −0.02 (0.38) | 0.96 | −12.9 (8.91) | 0.15 |
| Initial level and constant change parameters | ||||||
| Level cannabis use | 0.14 (0.26) | 0.59 | 0.84 (0.56) | 0.14 | 0.07 (0.30) | 0.81 |
| Level depression | 5.17 (0.26) | <0.01 | 6.52 (0.45) | <0.01 | 4.47 (0.17) | <0.01 |
| Change cannabis use | 2.41 (0.70) | <0.01 | −0.16 (1.75) | 0.92 | 6.05 (0.17) | 0.35 |
| Change depression | 0.07 (0.50) | 0.88 | −4.00 (1.75) | 0.01 | −1.47 (0.43) | <0.01 |
| Correlation between initial levels and change parameters | ||||||
| Level cannabis use with Change cannabis use | −0.18 (0.05) | <0.01 | 0.27 (0.12) | 0.03 | −0.53 (0.39) | 0.18 |
| Level depression with Change depression | −0.19 (0.32) | 0.55 | −0.66 (0.35) | 0.06 | −0.89 (0.04) | <0.01 |
| Level depression with Change cannabis use | 0.73 (0.12) | <0.01 | −0.85 (0.08) | <0.01 | 0.89 (0.12) | <0.01 |
| Level cannabis use with Level depression | −0.18 (0.05) | 0.02 | −0.11 (0.12) | 0.38 | −0.12 (0.06) | 0.06 |
| Level cannabis use with Change depression | 0.19 (0.14) | 0.18 | 0.45 (0.19) | 0.02 | 0.13 (0.07) | 0.09 |
| Change cannabis use with Change depression | −0.49 (0.29) | 0.09 | 0.84 (0.29) | <0.01 | −0.79 (0.13) | <0.01 |
| Variance of initial level and constant change parameters | ||||||
| Level cannabis use | 19.2 (1.45) | <0.01 | 12.8 (3.60) | <0.01 | 8.95 (1.45) | <0.01 |
| Level depression | 15.9 (1.06) | <0.01 | 23.7 (2.84) | <0.01 | 9.88 (0.47) | <0.01 |
| Change cannabis use | 4.17 (1.31) | <0.01 | 14.9 (7.12) | 0.04 | 24.8 (38.0) | 0.51 |
| Change depression | 0.71 (0.21) | <0.01 | 2.49 (2.70) | 0.36 | 1.05 (0.61) | 0.08 |
| Model Fit Criteria | ||||||
| χ 2 | 731.5 | 292.0 | 363.8 | |||
| df | 177 | 153 | 141 | |||
| CFI | 0.96 | 0.95 | 0.95 | |||
| TLI | 0.95 | 0.95 | 0.95 | |||
| RMSEA | 0.04 | 0.05 | 0.04 | |||
| SRMR | 0.06 | 0.08 | 0.07 | |||
Visual representation of effects.
To gain a more in-depth understanding, we provide visual representations of our final model where we explore what happens to depression symptoms during young adulthood when we force increases in cannabis use (Figure 2a). A hypothetical young adult starts with average levels (i.e., intercept values) of depression (score of 5.4) and cannabis use (average of ~1 day per month) at age 17. We then force increases in cannabis use by 3 additional days per month for each year from ages 18 to 21. As cannabis use increases to nearly 10 days per month by age 21, we see a slow and steady increase in depressive symptomology, peaking at age 22 (10 symptoms per month). As we force cannabis use back down to initial levels (reducing 3 days per month per year), by age 24, depressive symptoms remain higher than initial levels (score of 7.5).
Figure 2.
Effect of forced changes in cannabis use (2a) on depression and forced changes in depression on cannabis use (2b) from 17 to 24 years old for the whole sample. Predicted trajectories for a hypothetical young adult who begins with average levels of depression and cannabis use (i.e., intercept values) at age 17. We then provide “system shocks” to cannabis use to understand the effect on depression symptoms over time.
On the other side of this dynamic process, we explored what would happen to cannabis use if we forced increases in depression. As we forced increases in depression through age 21 (1 symptom increase per year; Figure 2b), cannabis use increased by very small, modest increments. As depression decreased (reducing 1 symptom per year), cannabis use returned to initial levels.
Within-Person Associations between Depression and Cannabis Use for ACEs+ and ACEs−
Table 2 provides final estimates of latent difference score models for ACEs+ and ACEs− individuals. Focusing on within-person parameters among ACEs+ individuals, results from our proportional coupling parameters reveal that greater prior levels of cannabis use are associated with decreasing depression (β = −0.18), which is not consistent with a substance-induced pathway. We also show support for a symptom-driven pathway, which indicates greater prior levels of depression symptoms are associated with positive change in cannabis use (increasing cannabis use; β = 0.68).
In contrast, the model for ACEs− indicated no significant proportional coupling parameters. However, results for the dynamic coupling parameters indicated that prior positive change in cannabis use (increasing use) is associated with positive change in depression symptoms (increasing depression). For example, individuals who experienced greater previous increases in cannabis use from 19 to 20 years old showed a more rapid increase in depression symptoms from 20 to 21 years old.
Visual representation of effects.
Using methods outlined above, we explore what happens to depression symptoms during adulthood when we force increases in cannabis use among those who report ACEs (Figure 3a) and those who do not (Figure 3b). Starting with ACEs+ (Figure 2a), a hypothetical young adult starts with average levels (i.e., intercept values) of depression (score of 6.5) and cannabis use (average of ~1 day per month) at age 17. As cannabis use increases through age 21, we see a slow and steady decrease in depressive symptomology. As we force cannabis use back down to initial levels, depression reaches its lowest point at age 24 (score of 4). An opposite pattern emerges for ACEs−. That is, in Figure 3b, as we force increases in cannabis use through age 21, depression slowly increases. In fact, even when cannabis use is forced to subside back to initial levels, depression symptoms still remain stably high, peaking at age 24 (score 6.9).
Figure 3.
Effect of forced changes in cannabis use on depression from 17 to 24 years old for ACEs+ and ACEs−. Predicted trajectories for a hypothetical young adult who begins with average levels of depression and cannabis use (i.e., intercept values) at age 17. We then provide “system shocks” to cannabis use to understand the effect on depression symptoms over time.
We also explored what would happen to cannabis use if we forced increases in depression. For ACEs+ (Figure 4a), forced increases in depression through age 21 (1 symptom increase per year) led to steady increases in cannabis use from ~1 day at age 17 to 4.8 days of use by age 22. However, as we forced depression symptoms back to initial levels, cannabis use did not decrease, but remained at modest levels at age 24 (~5 days). For ACEs−, we were unable to plot effects of forced changes in depression on cannabis use. Attempts to increase depression by any amount resulted in rapid negative values in cannabis use.
Figure 4.
Effect of forced changes in depression on cannabis use from 17 to 24 years old. Predicted trajectories for a hypothetical young adult who begins with average levels of depression and cannabis use (i.e., intercept values) at age 17. We then provide “system shocks” to depression symptoms to understand the effect on cannabis use over time. NOTE: no plot is shown for ACEs− given extreme sensitivity (large, negative values) of forced change in depression on change in cannabis use.
DISCUSSION
The goal of this study was to provide some clarity on the dynamic, developmental, interplay between cannabis use and depression symptomology by testing two directional associations: the symptoms-driven pathway and the substance-induced pathway. Further, we examined shared vulnerabilities in these directional associations by experience of ACEs. In contrast to what the symptom-driven hypothesis posits, in the full sample we showed that young adults who reported greater prior levels of depression symptoms at, say, 18 years old, tended to decrease their cannabis use as they got older. This is also depicted in our visual representation such that, as depression is forced to steadily increase, cannabis use remains unchanged (see Figure 2b). There has been mixed evidence for symptom-driven models with some reviews noting past year major depressive episodes are not robustly associated with greater odds of later cannabis use (6). Consistent with our results, Schoeler and colleagues note that a diagnosis of major depressive disorder at any point between ages 18 and 30 was associated with reduced frequency of cannabis use later in life (between 32 and 50). Our results also noted an opposite effect when assessing dynamic coupling parameters whereby individuals who evidenced greater increases in cannabis use between two prior ages (e.g., 18 and 19 years old) also reported greater increases in depressive symptoms between subsequent ages (19 and 20 years old). Again, this is noted in our visual representation of a prototypical young adult such that, as cannabis use is forced to increase, depression follows suit – and, further, remains high even after cannabis use is forced back to initial levels. This discrepancy may initially seem to conflate the directional evidence; however, our results are the first to show that effects of depression on subsequent cannabis use and vice versa may differ depending on whether the specified predictor is symptom severity at a single point in time as opposed to changes in symptoms over time. These lagged changes indicate that increases in cannabis use over the course of young adulthood is associated with rapid and stable increases in depression. One explanation of this could be a belief that cannabis use may alleviate already elevated depressive symptomology and, although this may alleviate symptoms in the short term (although we are unable to determine this), over the course of young adulthood, it appears that this is not the case, and depression increases and remains high. Overall, results provide a more nuanced characterization of the interplay between cannabis use and depressive symptoms over time and underscore the importance of considering longitudinal within-person changes in both cannabis use and depression, particularly during the key developmental periods of adolescence and young adulthood.
Our results also supported shared vulnerability models (21-23), noting different effects across those who have and have not experienced ACEs. One of the more striking differences is the effect of earlier cannabis use on later changes in depression. Similar to the overall sample, for those who have not experienced ACEs, as cannabis use increased we saw a steady increase in depression. However, for those who experienced ACEs, as cannabis use increased we saw a consistent decrease in depression. Interestingly, for those in the ACEs+ group, depression symptoms decreased to nearly zero following steady increases in cannabis use. These differences may be due to the wide range of effects that ACEs, or trauma in general, can have on the brain and body. For example, from a physiological perspective, the most disruptive form of stress response is a result of frequent and prolonged activation of the stress response system (e.g., Hypothalamic pituitary adrenocortical axis), often associated with long-term physiological and behavioral problems (24,25). Though preliminary, recent work indicates that there may be positive therapeutic effects of cannabis for those diagnosed with a psychological disorder, including depression (26-28). Some studies have also noted that cannabis use may mitigate the association between post-traumatic stress disorder (PTSD) and future depressive disorders (29), indicating cannabis may reduce the association between exposure to traumatic events (e.g., ACEs) and long-term psychopathology, such as depression. Although we cannot test this or other possible explanations, our results note differing associations between cannabis use and depression, dependent on experiences of adverse childhood experiences, that deserve further study.
Limitations and Conclusion
This study should be interpreted in light of several limitations. First, data are self-reported and thus results are potentially biased due to shared variance. Second, depressive symptoms were measured using a well-established screener for possible depressive disorder, but it is not a diagnostic measure. Third, although we have temporally ordered data, we cannot determine causality. Fourth, although we did address heterogeneity across childhood adversities, more research is needed to address subgroup differences by race/ethnicity, sexual and gender minority identities, and other shared vulnerabilities. Finally, while our study does represent one of the largest samples to test associations between depression and cannabis use, our subsample analyses by experiences of ACEs may be underpowered to detect effects.
Overall, the present study supports and extends our understanding of directional associations between depressive symptomatology and cannabis use. In particular, we show a nuanced set of results which support a dynamic interplay between depression and cannabis use. It is essential for clinicians to recognizing how pathways between cannabis use and depression symptoms may interact, especially among those who have and have not experienced ACEs. In particular, it seems that, overall, as young adults increase their cannabis use depression follows a steady increase to near clinical levels, however these increases in depression following cannabis use appear to vary by important shared vulnerabilities. Thus, clinicians working with young adults without prior childhood adversity should screen for cannabis use and potentially discuss alternative ways to cope with depressive symptoms. Though results show rapid decreases in depression following increases in cannabis use among those who have experienced childhood adversity, it is still important for clinicians to discuss both positive and negative effects of cannabis use on psychological well-being as individuals can respond differently to cannabis use. This is especially critical as recent reports of increased cannabis potency are associated with greater likelihood of onset of psychiatric symptomology (30). Future research should continue exploring within-person change and include important mechanisms that may link depression and cannabis use during young adulthood. Overall, our results highlight that the link between cannabis use and depression is complex with both positive and negative effects of cannabis use on depression, with important differences depending upon shared vulnerabilities.
Supplementary Material
Funding:
Work on this article was supported by three grants from the National Institute of Alcohol Abuse and Alcoholism (R01AA016577; R01AA020883; R01AA025848) to Elizabeth D’Amico.
Footnotes
Declaration of interest: none to declare
References
- 1.SAMHSA. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Rockville, MD; 2021. [Google Scholar]
- 2.Lev-Ran S, Roerecke M, Le Foll B, George TP, Mckenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med [Internet]. 2014;44(797–810). Available from: 10.1017/S0033291713001438 [DOI] [PubMed] [Google Scholar]
- 3.Garey L, Olofsson H, Garza T, Rogers AH, Kauffman BY, Zvolensky MJ. Directional Effects of Anxiety and Depressive Disorders with Substance Use: a Review of Recent Prospective Research. Curr Addict Reports. 2020. Sep 1;7(3):344–55. [Google Scholar]
- 4.Myers B, McLaughlin KA, Wang S, Blanco C, Stein DJ. Associations between childhood adversity, adult stressful life events, and past-year drug use disorders in the national epidemiological study of alcohol and related conditions (NESARC). Psychol Addict Behav [Internet]. 2014. [cited 2022 Jun 1];28(4):1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasan A, von Keller R, Friemel CM, Hall W, Schneider M, Koethe D, et al. Cannabis use and psychosis: a review of reviews. Eur Arch Psychiatry Clin Neurosci [Internet]. 2020. Jun 1 [cited 2022 May 25];270(4):403–12. [DOI] [PubMed] [Google Scholar]
- 6.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction [Internet]. 2003. Nov 1 [cited 2022 Jun 1];98(11):1493–504. [DOI] [PubMed] [Google Scholar]
- 7.Schoeler T, Theobald D, Pingault J-B, Farrington DP, Coid JW, Bhattacharyya S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. Psychol Med [Internet]. 2018. [cited 2022 Jun 1];48(13):2169–76. [DOI] [PubMed] [Google Scholar]
- 8.Rhew IC, Fleming CB, Stoep A Vander, Nicodimos S, Zheng C, Mccauley E. Examination of cumulative effects of early adolescent depression on cannabis and alcohol use disorder in late adolescence in a community-based cohort. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Womack SR, Shaw DS, Weaver CM, Forbes EE. Bidirectional Associations Between Cannabis Use and Depressive Symptoms From Adolescence Through Early Adulthood Among At-Risk Young Men. http://dx.doi.org/1015288/jsad201677287 [Internet]. 2016. Mar 22 [cited 2022 Jun 1];77(2):287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London-Nadeau K, Rioux C, Parent S, Vitaro F, Côté SM, Boivin M, et al. Longitudinal associations of cannabis, depression, and anxiety in heterosexual and LGB adolescents. J Abnorm Psychol. 2021. May 1;130(4):333–45. [DOI] [PubMed] [Google Scholar]
- 11.Berry D, Willoughby M. On the practical interpretabilty of cross-lagged panel models:Rethinking a developmental workhorse. Child Dev. 2016;0(0):1–21. [DOI] [PubMed] [Google Scholar]
- 12.Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements-Nolle K, Lensch T, Drake C, behaviors JP-A, 2022 undefined. Adverse childhood experiences and past 30-day cannabis use among middle and high school students: the protective influence of families and schools. Addict Behav [Internet]. 2022. [cited 2022 Jun 1];130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Kim K, Chartier KG, Wike TL, Mcdonald SE. Adverse childhood experience patterns, major depressive disorder, and substance use disorder in older adults. 2019; [DOI] [PubMed] [Google Scholar]
- 15.Yoon S, Shi Y, Yoon D, Pei F, Schoppe-Sullivan S, Snyder SM. Child Maltreatment, Fathers, and Adolescent Alcohol and Marijuana Use Trajectories. Subst Use Misuse [Internet]. 2020. Mar 2 [cited 2020 Nov 23];55(5):721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amico EJ, Tucker JS, Miles JNV., Zhou AJ, Shih RA, Green HD. Preventing Alcohol Use with a Voluntary After-School Program for Middle School Students: Results from a Cluster Randomized Controlled Trial of CHOICE. Prev Sci [Internet]. 2012. Aug 5 [cited 2016 Jul 28];13(4):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Amico E, Rodriguez A, Tucker J, Dunbar M, Pedersen E, Seelam R. Disparities in functioning from alcohol and cannabis use among a racially/ethnically diverse sample of emerging adults. Drug Alcohol Depend [Internet]. 2022. [cited 2022 Jun 1];234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med [Internet]. 1998. May [cited 2016 Oct 31];14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 19.Grimm KJ, An Y, Mcardle JJ, Zonderman AB, Resnick SM. Recent Changes Leading to Subsequent Changes: Extensions of Multivariate Latent Difference Score Models) Recent Changes Leading to Subsequent Changes: Extensions of Multivariate Latent Difference Score Models, Structural Equation Modeling: A Multidiscipli. Struct Equ Model A Multidiscip J [Internet]. 2012;19(2):268–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthén BO, Muthén LK. Mplus User’s Guide: Eigth Edition. Los Angeles, CA.; [Google Scholar]
- 21.Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and Other Illicit Drugs: Comorbid Use and Abuse/Dependence in Males and Females. Behav Genet [Internet]. 2004. May [cited 2020 Nov 23];34(3):217–28. [DOI] [PubMed] [Google Scholar]
- 22.Bulik CM, Prescott CA, Kendler KS. Features of childhood sexual abuse and the development of psychiatric and substance use disorders. Br J Psychiatry [Internet]. 2001. Nov [cited 2018 Aug 12];179:444–9. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin KA, Conron KJ, Koenen KC, Gilman SE, Arata CM, Langhinrichsen-Rohling J, et al. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med [Internet]. 2010. Oct 17 [cited 2016 Nov 4];40(10):1647–58. Available from: http://www.journals.cambridge.org/abstract_S0033291709992121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999. Jan;160(1):1–12. [DOI] [PubMed] [Google Scholar]
- 25.Iob E, Baldwin JR, Plomin R, Steptoe A. Adverse childhood experiences, daytime salivary cortisol, and depressive symptoms in early adulthood: a longitudinal genetically informed twin study. Transl Psychiatry 2021 111 [Internet]. 2021. Aug 5 [cited 2022 May 25];11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: A longitudinal study. J Affect Disord. 2015. Feb 1;172:211–8. [DOI] [PubMed] [Google Scholar]
- 27.Feingold D, Hoch E, Weinstein A, Hall W. Editorial: Psychological Aspects of Cannabis Use and Cannabis Use Disorder. Front Psychiatry [Internet]. 2021. Nov 5 [cited 2022 Jun 1];12:789197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen K, Weizman A, Weinstein A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin Pharmacol Ther ∣ Vol [Internet]. 2019;105. [DOI] [PubMed] [Google Scholar]
- 29.Lake S, Kerr T, Buxton J, Walsh Z, Marshall B, Wood E, et al. Does cannabis use modify the effect of post-traumatic stress disorder on severe depression and suicidal ideation? Evidence from a population-based cross-sectional study of Canadians. J Psychopharmacol [Internet]. 2020;34(2):181–8. [DOI] [PubMed] [Google Scholar]
- 30.Wilson J, Freeman TP, Mackie CJ. Effects of increasing cannabis potency on adolescent health. Lancet Child Adolesc Heal. 2019. Feb 1;3(2):121–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.