Table 1.
Anti-angiogenic drugs approved by FDA for clinical treatment
| Drugs | Targets | Indications | Companies | Adverse effects |
|---|---|---|---|---|
| Monoclonal antibodies | ||||
| Bevacizumab (Avastin®) | VEGF-A |
CRC in 2004, NSCLC in 2006, RCC in 2009, GBM in 2009, CC in 2014 |
Genentech/Roche | Arterial or venous, back pain, dry skin, exfoliative dermatitis gastrointestinal perforation, headache, hemorrhage, hypertension, lacrimation disorder, poor wound healing, proteinuria, rhinitis, and taste alteration, thrombosis |
| Ranibizumab (Lucentis®, RG-6321) | VEGF-A |
wAMD in 2006, DME in 2015, DR in 2017, Myopic choroidal neovascularization in 2017 |
Genentech/Roche | Conjunctival hemorrhage, endophthalmitis, eye infection, eye pain, floaters, increased intraocular pressure, rhegmatogenous retinal detachment, and retinal hemorrhage |
| Ramucirumab (Cyramza®) | VEGFR-2 | NSCLC in 2014, Advanced GC in 2014, GEJ adenocarcinoma in 2014, metastatic CRC in 2015 | Genentech and Eli Lilly | Abdominal pain, thrombocytopenia, anorexia, arthralgia, constipation, cough, diarrhea, dyspnea, epistaxis, fatigue, headache, hypertension, leucopenia, nausea, neutropenia, peripheral edema, proteinuria, upper respiratory tract infection, and vomiting |
| Olaratumab (Lartruvo®) | PDGFR-α | STS in 2016 | ImClone/Eli Lilly | Appetite, abdominal pain, alopecia, diarrhea, decreased fatigue, headache, neuropathy, musculoskeletal pain, mucositis, nausea, and vomiting |
| Bevacizumab-awwb (Mvasi®) | VEGF | CRC, NSCLC, RCC, GBM, and CC in 2017 | Amgen | Altered taste, arterial and venous thromboembolic events, bleeding, dry skin, epistaxis, exfoliative dermatitis, headache, hypertension, hypertension, infusion-related reactions, lacrimation disorders, ovarian failure, perforation or fistula, post-reversible encephalopathy syndrome, proteinuria, proteinuria, and rhinitis |
| Oligonucleotide aptamers | ||||
| Pegaptanib (Macugen®) | VEGF-A165 | wAMD in 2004 | Eyetech/Pfizer | Endophthalmitis and retinal detachment |
| Recombinant fusion proteins | ||||
| Aflibercept (Eylea®) | VEGF-A, VEGF-B, PlGF |
wAMD in 2011 CRC in 2012 DME in 2015 DR in 2019 |
Regeneron | Cataracts, conjunctival hemorrhage, decreased vision, eye pain, floaters, increased intraocular pressure, and vitreous detachment |
| ziv-Aflibercept (Zaltrap®) | VEGF-A, VEGF-B, PlGF | CRC in 2012 | Sanofi and Regeneron | Abdominal pain, bleeding, decreased appetite, decreased ejection fraction, diarrhea, dyspnea, epigastric pain, fatigue, fatigue, gastrointestinal perforation, headache, heart failure, hypertension, impaired wound healing, infection, leukopenia, nephrotic syndrome, neutropenia, osteonecrosis of the lower jaw, proteinuria, severe diarrhea, stomatitis, thrombocytopenia, and weight loss |
| mTOR inhibitors | ||||
| Temsirolimus (Torisel®) | mTOR | RCC in 2007 | Wyeth | Acute renal failure, asthenia, edema, elevated aspartate aminotransferases, hyperlipidemia, hypersensitivity, interstitial pneumonia, intestinal perforation, lymphopenia, mucositis, nausea, rash, thrombocytopenia |
| Everolimus (RAD001, Afinitor®) | mTOR |
RCC in 2009 SEGA in 2010 pNET in 2011 HER2- BC |
Novartis | Canker sores, increased heart rate, paronychia, rash, swollen and painful gums, tiredness, and tongue ulcers |
| Immunomodulatory agents | ||||
|
Thalidomide (Thalomid®)
|
VEGF-A, TNF, NF-κB | MM in 2006 | Celgene | Abdominal pain, constipation, dizziness, drowsiness, dry oral mucosa, facial puffiness, nausea, rash, teratogenic, and tiredness |
|
Lenalidomide (Revlimid®)
|
VEGF-A, TNF, NF-κB |
MM in 2006 MCL in 2013 |
Celgene | Anemia, diarrhea, fatigue, headache, loss of appetite, low back pain, neo-malignant neoplasms, neutropenia, rash, renal insufficiency, thrombocytopenia, and thrombotic complications |
| Tyrosine kinase inhibitors | ||||
|
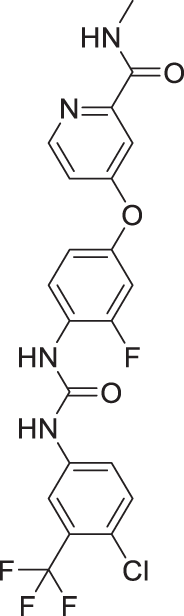
Sorafenib (Nexavar®, BAY-439006)
|
VEGFR-1/-2/-3, c-Kit, Flt-3, PDGFR-β, Raf, Ret |
RCC in 2005 HCC in 2007 DTC in 2013 TC in 2014 |
Bayer and Onyx/Amgen |
Abdominal pain, alopecia, decreased appetite, diarrhea, fatigue, hand-foot skin reaction, hypertension, nausea, rash, and weight loss |
|
Sunitinib (Sutent®, SU11248)
|
VEGFR-1/-2/-3, Flt-3, c-Kit, Ret, PDGFR-α/-β, CSF-1R |
RCC in 2006 GIST in 2006 pNET in 2011 |
Sugen/Pfizer | Abdominal pain, anorexia, asthenia, diarrhea, dysgeusia, dyspepsia, fatigue, hypertension, mucositis, nausea, skin discoloration, stomatitis, and thrombocytopenia |
|
Pazopanib (Votrient®, GW-786034)
|
VEGFR-1/-2/3, c-Kit, PDGFR-α/-β, |
RCC in 2009 STS in 2012 |
GlaxoSmithKline | Anorexia, diarrhea, fatigue, fatigue, hair color changes, nausea, vomiting, and weight loss |
|
Vandetanib (Caprelsa®, ZD6474)
|
VEGFR-2, VEGFR-3, EGFR, Ret | MTC in 2011 | AstraZeneca | Diarrhea, headache, rash, hypertension, nausea, and QTc prolongation |
|
Regorafenib (Stivarga®)
|
VEGFR-1/-2/-3, c-Kit, PDGFR-β, Ret, Raf-1, bRaf, FGFR-1, Tie-2 |
CRC in 2012 GIST in 2013 HCC in 2017 |
Bayer | Anorexia, diarrhea, fatigue, hand-foot skin reaction, hypertension, and oral mucositis |
|
Axitinib (Inlyta®, AG013736)
|
VEGFR-1/-2/3, c-Kit, PDGFR-α, PDGFR-β | RCC in 2012 | Pfizer | Asthenia, constipation, decreased appetite, diarrhea, dysphonia, fatigue, hand-foot syndrome, hypertension, nausea, vomiting, and weight decreased |
|
Ponatinib (Iclusig®)
|
VEGFRs, PDGFRs, EPHs, FGFRs, ABL, Src, Ret, LYN, LCK, c-Kit, HCK, FYN, FRK, c-FMS, FGR, BLK |
CML in 2012 Ph+ AML in 2012 |
Ariad/Takeda | Abdominal pain, arthralgia, dermatitis, dry skin, fatigue, increased lipase, nausea, rash, and thrombocytopenia |
|
Cabozantinib (Cometriq®, BMS-907351)
|
VEGFR-2, c-Met, c-Kit, Ret, Flt-3, Tie-2, AXL, RON |
MTC in 2013 RCC in 2016 HCC in 2019 |
Exelixis | Abdominal pain, constipation, decreased appetite, decreased weight, diarrhea, dysgeusia, fatigue, hair color changes, hypertension, nausea, oral pain, palmar-plantar erythrodysesthesia syndrome, and stomatitis |
|
Apatinib (Aitan®)
|
VEGFR-2, Src, c-Kit | GC in 2014 | Hengrui Medicine | Fatigue, gastrointestinal bleeding, granulocytopenia, hand-foot syndrome, hoarseness, hypertension, leukopenia, proteinuria, and thrombocytopenia |
|
Nintedanib (Ofev®, BIBF1120)
|
VEGFRs, FGFRs, PDGFRs, Flt-3, LCK, LYN, Src | NSCLC in 2015 |
Boehringer and Ingelheim |
Bleeding, decreased appetite, diarrhea, electrolyte imbalance, mucositis, nausea, neutropenia, peripheral neuropathy, rash, and vomiting |
|
Lenvatinib (Lenvima®, E7080)
|
VEGFRs, PDGFRs, Ret, c-Kit, FGFRs |
DTC and TC in 2015 RCC in 2016 HCC in 2018 Endometrial Carcinoma in 2019 |
Eisai | Abdominal pain, arthralgia, decreased appetite, decreased weight, diarrhea, dysphonia, fatigue, headache, hypertension, myalgia, nausea, proteinuria, stomatitis, and vomiting |
ALL acute lymphoblastic leukemia, BC breast cancer, BTC biliary tract cancer, CC cervical cancer, CML chronic myeloid leukemia, CRC colorectal cancer, CSF colony-stimulating factor, DME diabetic macular edema, DR diabetic retinopathy, DTC differentiated thyroid cancer, EC esophageal cancer, GEJ gastroesophageal junction, GBM glioblastoma, GC gastric cancer, GIST gastrointestinal stromal tumor, HCC hepatocellular carcinoma, HER2 human epidermal growth factor receptor 2, HNSCC head and neck squamous cell carcinoma, MCL mantle cell lymphoma, MM multiple myeloma, MTC medullary thyroid cancer, mTOR mammalian target of rapamycin, NSCLC non-small cell lung cancer, Ph+ AML Philadelphia chromosome-positive acute myeloid leukemia, pNET pancreas neuroendocrine tumor, RCC renal cell carcinoma, SEGA subependymal giant cell astrocytoma, STS soft tissue sarcoma, TC thyroid cancer, TNBC triple-negative breast cancer, wAMD wet age-related macular degeneration