Table 2.
Current status of potential angiogenic inhibitors in clinical development
| Agents | Targets | Phase | Status | Conditions or diseases | Trial ID |
|---|---|---|---|---|---|
| Antibodies | |||||
| Bemarituzumab (FPA144) | FGFR-2 | I | Not yet recruiting | Solid tumors (unspecified) | NCT05325866 |
| III | Recruiting | GC or CEJ adenocarcinoma | NCT05052801 | ||
| JY-025 | VEGFR-2 | II/III | Not yet recruiting | NSCLC with EGFR 19 exon deletion or 21 exon mutation | NCT04874844 |
| BAT-5906 | VEGFR | III | Not yet recruiting | wAMD | NCT05439629 |
| II | Completed | wAMD | NCT05141994 | ||
| I/II | Recruiting | DME | NCT04772105 | ||
| Olinvacimab | VEGFR-2 | II | Recruiting | Metastatic TNBC | NCT04986852 |
| I/II | Not yet recruiting | Metastatic CRC who failed two prior standard chemotherapies | NCT04751955 | ||
| Ak109 | VEGFR-2 | I/II | Recruiting | Advanced solid tumor | NCT05142423 |
| I/II | Recruiting | Advanced gastric adenocarcinoma or GEJ adenocarcinoma | NCT04982276 | ||
| I | Unknown | Advanced solid tumors | NCT04547205 | ||
| CTX-009 (ABL001) | VEGF-A, Dll-4 | I/II | Recruiting | Advanced or metastatic solid tumors; unresectable advanced, metastatic or recurrent BTC | NCT04492033 |
| NOV-1105 (YYB-101) | HGFR | I/II | Recruiting | Metastatic or recurrent CRC | NCT04368507 |
| MCLA-129 | c-Met, EGFR | I/II | Recruiting | Advanced NSCLC or other solid tumors | NCT04930432 |
| I/II | Recruiting | Metastatic or advanced NSCLC, HNSCC or other solid tumors | NCT04868877 | ||
| SYD-3521 (BYON3521) | c-Met | I | Recruiting | Locally advanced or metastatic solid tumors | NCT05323045 |
| VRDN-001 | IGF-1 | I/II | Recruiting | TED | NCT05176639 |
| Oligonucleotide agents | |||||
| IGV-001 | – | II | Not yet recruiting | GBM or GBM multiforme | NCT04485949 |
| Anti-angiogenic fusion proteins | |||||
| 9MW-0813 | VEGFR | III | Recruiting | DME | NCT05324774 |
| I | Completed | DME | NCT05324592 | ||
| Tyrosine kinase inhibitors | |||||
|
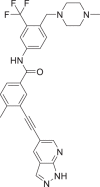
Surufatinib
|
VEGFR-1/-2/-3, CSF1R, FGFR-1 | II | Recruiting | Advanced CRC who failed front-line anti-angiogenic TKI therapy | NCT05372198 |
| II | Recruiting | Advanced HCC | NCT05171439 | ||
| II | Recruiting | HR+ unresectable metastatic BC refractory to endocrine therapy | NCT05186545 | ||
| II | Recruiting | High-grade advanced-neuroendocrine neoplasm | NCT05165407 | ||
| II | Not yet recruiting | Inoperable or metastatic advanced intrahepatic cholangiocarcinoma (ICC) | NCT05236699 | ||
| II | Not yet recruiting | OC with platinum-resistance and received prior PARP inhibitors | NCT05494580 | ||
| II | Not yet recruiting | Advanced gastric adenocarcinoma or GEJ adenocarcinoma | NCT05235906 | ||
|
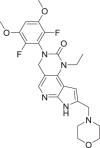
Avapritinib (BLU-285)
|
PDGFR-α, c-Kit | - | Approved | Locally advanced unresectable or metastatic GIST | NCT03862885 |
| IV | Active, not recruiting | GIST | NCT04825574 | ||
| II | Recruiting | Locally advanced or metastatic malignant solid tumors with c-Kit or PDGFR-α mutation-positive | NCT04771520 | ||
| II | Recruiting | Chinese patients with GIST | NCT05381753 | ||
| II | Active, not recruiting | Indolent systemic mastocytosis | NCT03731260 | ||
| I/II | Recruiting | Solid tumors with mutations in c-Kit or PDGFR-α, or gliomas with the H3K27M mutation | NCT04773782 | ||
| I/II | Active, not recruiting | Chinese subjects with unresectable or metastatic GIST | NCT04254939 | ||
| I | Recruiting | Metastatic or unresectable GIST, recurrent gliomas, or other c-Kit mutant tumors | NCT04908176 | ||
|
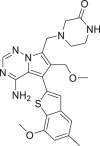
Olverembatinib (GZD824)
|
Bcr-Abl, c-Kit | III | Recruiting | CML in chronic phase who are resistant and/or intolerant to at least two second-generation tyrosine kinase inhibitors | NCT05311943 |
| II | Recruiting | Myeloproliferative neoplasms, ALL or AML with FGFR1 rearrangement | NCT05521204 | ||
| II | Recruiting | Advanced CML | NCT05376852 | ||
| II | Not yet recruiting | Ph+ ALL | NCT05466175 | ||
| I | Not yet recruiting | Relapsed or refractory Ph+ ALL | NCT05495035 | ||
|
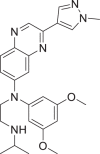
Pemigatinib
|
FGFR-1/-2/-3 | III | Recruiting | Unresectable or metastatic cholangiocarcinoma with FGFR2 rearrangement | NCT03656536 |
| II | Completed | Advanced/Metastatic or surgically unresectable cholangiocarcinoma with FGFR2 translocations who failed previous therapy | NCT02924376 | ||
| II | Recruiting | Previously treated GBM or other primary central nervous system tumors with FGFR1–3 alterations | NCT05267106 | ||
| II | Recruiting | GBM, or other primary CNS tumors, or adult-type diffuse gliomas with FGFR mutation | NCT05267106 | ||
| II | Recruiting | Advanced NSCLC with FGFR alterations who have failed standard therapy | NCT05287386 | ||
| II | Recruiting | Advanced GC or CRC with FGFR alterations who have failed standard therapy | NCT05202236 | ||
| II | Recruiting | HER2 negative advanced BC with FGFR 1–3 alterations who have failed standard therapy | NCT05560334 | ||
| II | Recruiting | Advanced gastrointestinal cancer (excluding BTC) with FGFR 1–3 alterations who have failed standard therapy | NCT05559775 | ||
| II | Recruiting | Relapsed or refractory advanced NSCLC with FGFR mutation | NCT05253807 | ||
| II | Active, not recruiting | Advanced or unresectable CRC with FGFR mutation | NCT04096417 | ||
| II | Active, not recruiting | Advanced, metastatic or unresectable cholangiocarcinoma | NCT04256980 | ||
|
Futibatinib
|
FGFR-1/-2/-3/-4 | III | Recruiting | Advanced, metastatic, or recurrent unresectable cholangiocarcinoma harboring FGFR2 gene rearrangements | NCT04093362 |
| III | Recruiting | Advanced or metastatic STS | NCT03784014 | ||
| II | Recruiting | Advanced or metastatic HCC with FGF19 positive | NCT04828486 | ||
| II | Recruiting | Advanced/metastatic GC or GEJ cancer, myeloid or lymphoid neoplasm, or other solid tumors with FGFR1 mutation | NCT04189445 | ||
| I/II | Recruiting | Advanced NSCLC, or other advanced or metastatic solid tumors with KRas mutation | NCT04965818 | ||
| I/II | Recruiting | HER2 mutated NSCLC or other advanced solid tumors | NCT05532696 | ||
|
Rogaratinib
|
FGFR-1/-2/-3/-4 | II/III | Completed | Locally advanced or metastatic urothelial carcinoma with FGFR-positive | NCT03410693 |
| II | Completed | Cancer (unspecified) | NCT04125693 | ||
| II | Recruiting | Advanced GIST, STS | NCT04595747 | ||
| II | Active, not recruiting | Pretreated advanced SQCLC | NCT03762122 | ||
| I | Completed | FGFR positive refractory, locally advanced or metastatic solid tumors | NCT03788603 | ||
| I | Recruiting | Metastatic FGFR1/2/3 positive, hormone receptor positive BC | NCT04483505 | ||
|
Erdafitinib
|
FGFR-1/-2/-3/-4 | IV | Recruiting | Bladder cancer with FGFR mutation | NCT05052372 |
| II | Recruiting | Recurrent non-invasive bladder cancer with FGFR3 mutation | NCT04917809 | ||
| II | Recruiting | NSCLC with FGFR genetic alterations | NCT03827850 | ||
| II | Recruiting | Advanced solid tumors with FGFR alterations | NCT04083976 | ||
| II | Active, not recruiting | Advanced urothelial cancer with selected FGFR aberrations | NCT05564416 | ||
| I/II | Recruiting | Relapsed refractory multiple myeloma | NCT03732703 | ||
| I | Recruiting | Bladder cancer with FGFR genetic alterations | NCT05316155 | ||
| I | Recruiting | Metastatic urothelial carcinoma with alterations in FGFR 2/3 genes | NCT04963153 | ||
|
Telatinib
|
PDGFR-β, c-Kit, VEGFR |
II | Unknown | Advanced HER2 negative advanced gastric or GEJ adenocarcinoma | NCT03817411 |
| II | Recruiting | Advanced GC, GEJ adenocarcinoma, or HCC | NCT04798781 | ||
|
Tepotinib
|
c-Met | II | Recruiting | Solid tumors with Met amplification or Met exon 14 skipping mutation | NCT04647838 |
| II | Recruiting | Advanced or metastatic NSCLC with Met amplifications | NCT03940703 | ||
| I/II | Recruiting | Advanced NSCLC with Met mutation | NCT04739358 | ||
| I/II | Recruiting | advanced GC, GEJ cancer with Met amplified or Met exon 14 alternated | NCT05439993 | ||
| I | Completed | Patients with hepatic impairment | NCT03546608 | ||
| I | Not yet recruiting | Brain tumors with Met alternations | NCT05120960 | ||
ALL acute lymphoblastic leukemia, AML acute myeloid leukemia, BC breast cancer, BTC biliary tract cancer, CML chronic myeloid leukemia, CRC colorectal cancer, DME diabetic macular edema, GEJ gastroesophageal junction, GBM glioblastoma, GC gastric cancer, GIST gastrointestinal stromal tumor, HCC hepatocellular carcinoma, HER2 human epidermal growth factor receptor 2, HNSCC head and neck squamous cell carcinoma, NSCLC non-small cell lung cancer, PARP poly ADP-ribose polymerase, Ph+ AML Philadelphia chromosome-positive acute myeloid leukemia, RCC renal cell carcinoma, SQCLC squamous-cell non-small cell lung cancer, STS soft tissue sarcoma, TNBC triple-negative breast cancer, TED thyroid eye disease, wAMD wet age-related macular degeneration