Fig. 5.
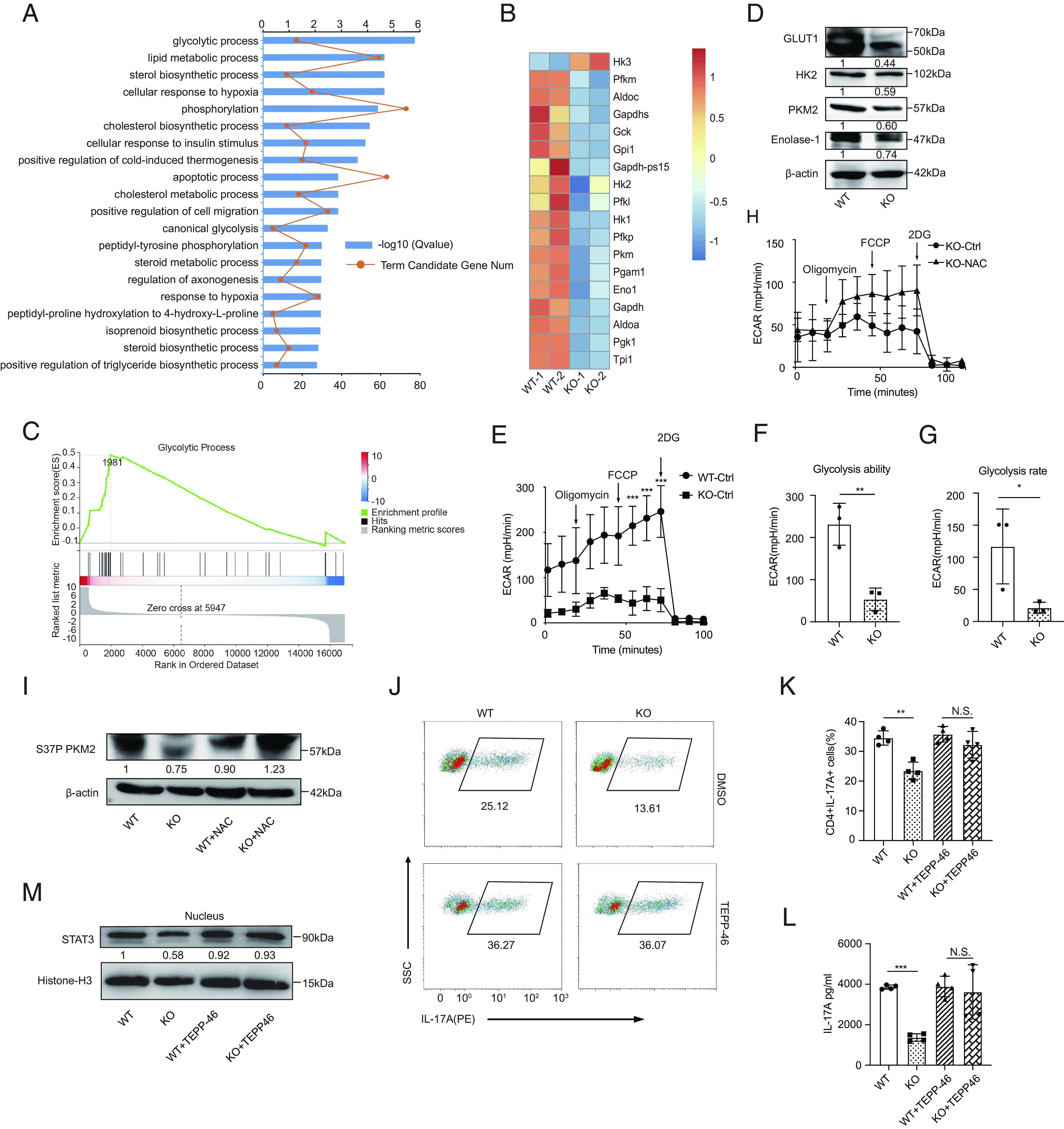
ROS accumulation in Prak-deficient Th17 cells inhibits glycolysis and STAT3 phosphorylation in a PKM2-dependent way. (A–C) RNA-seq was performed with WT and KO Th17 cells (n = 2). Gene oncology (GO) analysis of differentially expressed genes (A). Heatmap of glycolysis-related, differentially expressed genes (B). GSEA analysis of genes involved in glycolytic process (C). (D) Verification of the differential expression of HK2, Glut1, PKM2, and Enolase 1 at protein level by Western blotting (n = 3). (E–G) Extracellular acidification rate (ECAR) assay was performed with WT and KO Th17 cells (E). Glycolysis ability and glycolysis rate are shown in F and G, respectively. (H) ECAR assay of KO Th17 cells following treatment with NAC or solvent (n = 3). (I) Immunoblot of S37P PKM2 in WT and KO Th17 cells generated in the presence or absence of NAC (n = 3). (J–M) Th17 cells were generated with the addition of TEPP-46 or isovolumetric DMSO. Representative dot plots showing intracellular staining of IL-17A (J). Frequencies of IL-17A+ cells (J) (n = 4). Protein level of IL-17A in the culture supernatant (L) (n = 4). Immunoblot of nuclear STAT3, densitometry performed using histone-H3 as loading control (n = 3). The statistics was performed using Student’s t test. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; N.S. not significant.
