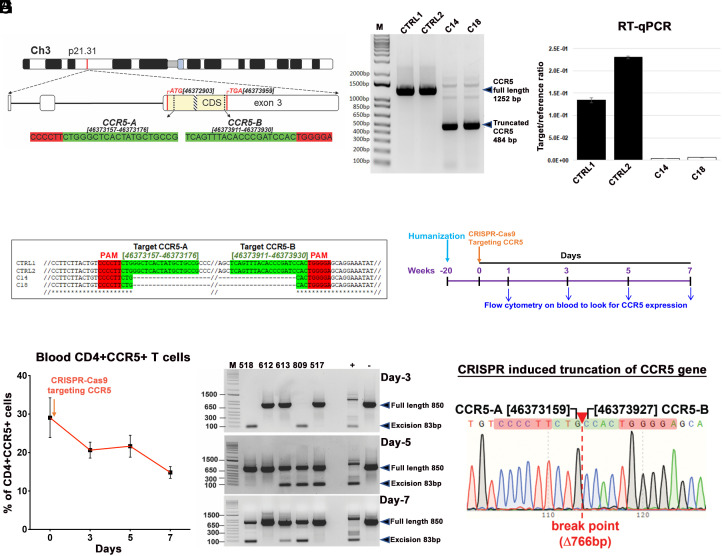
Fig. 1.
Guide-RNA location, CCR5 excision in vitro and in humanized mouse studies. (A) Chromosomal location and coordinates of CRISPR gRNA target sequences in the human CCR5 gene. The CCR5 coding sequence (CDS) is highlighted in yellow, positions of start and stop codons are in the red, the position of D32 mutation is shown as a patterned box, gRNA target sequences are highlighted in green, and PAMs in red. (B) Agarose gel analysis of PCR genotyping of CRISPR-Cas9-mediated cleavage of CCR5 gene. Genomic DNAs from two control (CTRL1 and CTRL2) and two CCR5 knockout (C14 and C18) TZM-bl single-cell clones were used as PCR template. (C) CCR5 mRNA expression in knockout clones was checked by reverse-transcription-qPCR from two controls and two knockout clones. (D) Alignment of Sanger sequencing results confirming CRISPR-induced truncation of CCR5. (E) Schematic illustration to look for CCR5 expression in human immune cells at different time points after a single IV injection of AAV6-CRISPR-Cas9-targeting CCR5 gene, in healthy humanized mice (n = 5). (F) Flow cytometric analysis of peripheral blood examined presence of CD3+CD4+CCR5+ T cells at 0, 3, 5, and 7 d after a single injection. Gating strategy was human CD45CD3CD4CCR5. Data are expressed as mean ± SEM. (G) CCR5 excision analysis on blood cells after single AAV6-CRISPR-Cas9 injection on the same humanized mice at different time points as described in E and F. The full-length and truncated bands are pointed in arrows. (H) Representative result of Sanger sequencing of the bands from humanized mice from F (mouse #809, day 3), confirming CRISPR-induced truncation of CCR5 gene.