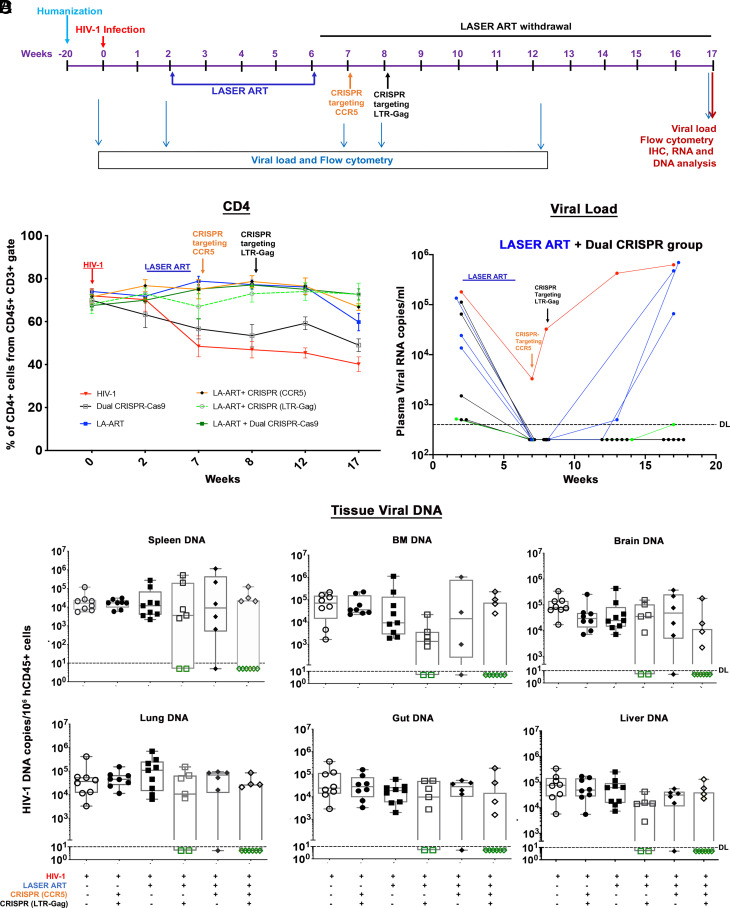
Fig. 2.
Viral and human immune profiles from LASER ART and CRISPR-Cas9 treatments of HIV-1-infected humanized mice. (A) Study scheme showing the timing of NSG-humanized mouse generation, HIV-1 infection, ART and single or dual CRISPR treatments to respective groups. After 2 wk of infection and confirmation of VL, mice were given intramuscular (IM) doses with 45 mg/kg NMCAB and NRPV and 40 mg/kg NM3TC, NMABC. Treatment was for 4 wk, followed by a single IV dose of AAV6-CRISPR-Cas9 CCR5, at week 7 and a second IV dose of AAV9-CRISPR-Cas9 LTR-Gag at week 8. Animals had antiretroviral medicines stopped for 11 wk at the time of sacrifice. (B) Evaluation of human CD45+CD3+CD4+ T cell numbers in humanized mice by flow cytometry tests on 0, 2, 7, 8, 12, and 17 wk postinfection. (Red line—HIV-1, Black—HIV+ dual CRISPR, blue—HIV+LASER ART, orange—HIV+LASER ART+CRISPR(CCR5), broken green—HIV+LASER ART+CRISPR(LTR-Gag) and solid green line indicates—HIV+LASER ART+ dual CRISPR group). (C) Plasma viral load assessment by determining viral RNA copies of individual animals assayed at 2, 7, 8, 12, and 17 wk after HIV-1ADA infection from the LASER ART and dual CRISPR treatment group. One animal represented in green showed viral RNA at the detection limit at study end. (D) HIV-1 DNA was measured by semi-nested real-time qPCR assays. The data represent mean ± SEM for each group. Analyses were performed from spleen, BM, gut, brain, liver, and lung tissues in each of the treatment groups. Six out of ten animals with LASER ART+ dual CRISPR treatments, 2/7 in LASER ART + HIV CRISPR, and 1/6 in LASER ART + CCR5 CRISPR showed complete viral elimination from all analyzed tissues.