FIGURE 5.
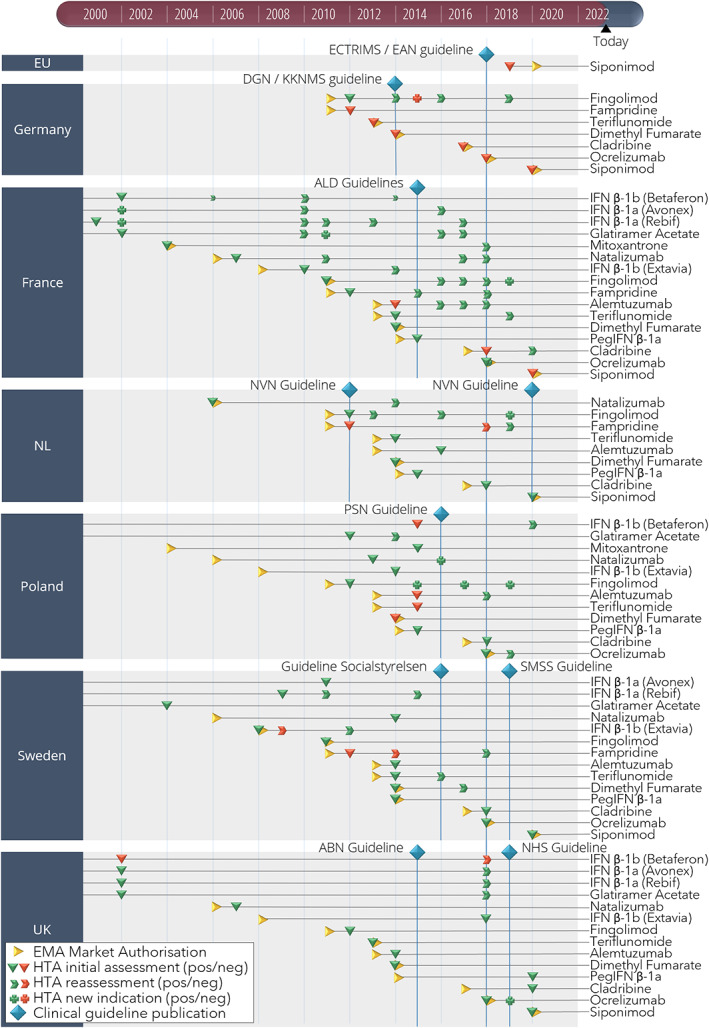
Market authorization, health technology assessment (HTA), and clinical guideline (CG) events for multiple sclerosis (MS) treatments in six countries. A timeline showing the events of market authorization, HTA assessment, and CG publication for MS treatments in six European countries between 2000 and 2021. ABN, Association of British Neurologists: revised guidelines for prescribing disease‐modifying treatments in multiple sclerosis; ALD, Haute Autorité de Santé Actes et prestations, affection de longue durée, Sclérose en plaques; DGN/KKNMS, Leitlinie zur Diagnose und Therapie der MS; ECTRIMS/EAN, guideline on the pharmacological treatment of people with multiple sclerosis; EMA, European Medicines Agency; EU, European Union; HTA, health technology assessment; NHS, National Health Service Treatment Algorithm for Multiple Sclerosis Disease‐Modifying Therapies; NL, the Netherlands; NVN, Nederlandse Vereniging voor Neurologie, Ziektemodulerende Behandeling van Multiple Sclerose bij volwassenen, Addendum bij de richtlijn Multiple Sclerose; PSN, Leczenie stwardnienia rozsianego Zalecenia Polskiego Towarzystwa Neurologicznego; Socialstyrelsen, Nationella riktlinjer Socialstyrelsen, Vård vid multipel skleros och Parkinsons sjukdom; SMSS, Svenska MS‐sällskapet, Läkemedel; UK, United Kingdom.
