Abstract
Objective: Granulomatosis with polyangiitis (GPA), formerly known as Wegner’s granulomatosis, is a rare vasculitic syndrome classified under Anti-Neutrophilic Cytoplasmic Autoantibody (ANCA)-associated vasculitides, which is fatal if untreated. The mainstay of treatment consists of immunosuppression using a combination of corticosteroids with either rituximab (RTX) or cyclophosphamide (CYC). We aimed to compare the 4-year clinical outcomes between patients with GPA receiving CYC and RTX as remission induction.
Methods: In this retrospective cohort, we used patient data from 92 patients with GPA at two large teaching hospitals and a private clinic in Isfahan, Iran. The patients were classified based on the medication they received for remission induction into RTX and CYC groups. The main outcomes were rate of death and relapse, disease activity assessed based on the Birmingham Vasculitis Activity Score (BVAS), disease-related complications, laboratory markers, and adverse-drug-reactions.
Results: Fifty-three (57.6%) patients received CYC, whereas 39 (42.4%) received RTX. The mean duration of follow-up was 3.6 (±2) years. Most of patients (70%) had a successful remission, while 20.7% experienced a relapse and 8.7% of patients died. The rate of death and relapse did not differ between the RTX and CYC groups. Disease-related complications involved an insignificantly higher proportion of patients in the CYC (12/53) group than the RTX (4/39) group. Patients in both groups showed a significant decrease in BVAS during follow-ups irrespective of the medication exposure. The rate of adverse events was similarly low (n = 1) in both groups.
Conclusion: RTX and CYC were similar in inducing remission and reducing adverse clinical outcomes among patients with GPA with acceptable side effect profiles.
Keywords: Wegener, granulomatosis with polyangiitis, Iran
Main Points
In recent years, cyclophosphamide has been used as the main induction treatment in patients with Wegener's Granulomatosis disease, but in recent years it has been shown that Rituximab can be equally effective for treatment of this condition.
Rituximab could be as effective as Cyclophosphamide in the induction phase of Wegener's granulomatous patients treatment.
Rituximab seems to have fewer side effects than cyclophosphamide. However, there was no statistically significant difference between them in our study.
Brimingham vasculitis activity score (BVAS) was significantly reduced in both groups of patients receiving rituximab and cyclophosphamide, although there was no statistically significant difference.
Introduction
Granulomatosis with polyangiitis (GPA), formerly known as Wegener’s granulomatosis, is a rare ANCA-associated necrotizing systemic vasculitis affecting small to medium vessels.1–3 Clinically, the respiratory tract and the kidneys are the most commonly involved, but involvement of pulmonary and ocular capillaries are also common.4 The treatment is initiated according to the patient’s condition; as a rapid and (preferably) lasting remission is aimed with patients who are in a life- or organ-threatening clinical course, while a more gradual immunosuppression is intended with patients who are not under such serious circumstances. The treatment is usually a two-stepped approach, consisting of “remission induction” and “remission maintenance.”5 It is recommended to induce remission in the case of life-/organ-threatening ANCA-associated vasculitides using a combination of glucocorticoids and either cyclophosphamide (CYC) or rituximab (RTX).6 To maintain remission, the European League Against Rheumatism (EULAR) guideline recommends low-dose glucocorticoids combined with either azathioprine, RTX, methotrexate, or mycophenolate mofetil.6
B-cells are frequently shown to be important in autoimmune conditions not only as producers of autoantibodies but also as mediators that cause, help initiate, and/or let it continue, for example, activation of T-cells.2,5,7 CYC benefits GPA patients through its B-cell-suppressing features.8–10 Also, a B-cell-count closer to normal measures has been correlated with relapse in GPA patients.3 Before some follow-up studies reveal considerable side effects for CYC in 1990,11 its combination with glucocorticoids was the major treatment regimen for inducing remission.12 RTX, an anti-CD20 monoclonal antibody first approved in 1997 to treat relapsed lymphomas,5 has been successfully employed to treat various autoimmune conditions,5,13,14 as well as GPA (first in 2001).15 It has been shown that RTX can deliver therapeutic benefits not inferior to CYC in the case of treating GPA, while saving patients from the numerous side effects potentially imposed on those treated using CYC, but according to EULAR, it is slightly less strongly recommended as a “first” induction therapy option compared with CYC.6 In the present study, we aimed to compare CYC to RTX in terms of long-term clinical efficacy as a remission induction therapy in an Iranian population.
Methods
Design and participants
We conducted a retrospective cohort study based on data collected from the files of patients with c-ANCA(PR3)-positive GPA who were being treated at Al-Zahra and Khorshid hospitals as well as a private rheumatology clinic from March 20, 2016 to September 22, 2020. The follow-up period was 4 years and included patients who were diagnosed with and treated for GPA with either CYC or RTX for 6 months from 2016 to February 2020. The patients were diagnosed based on American College of Rheumatology (ACR) criteria for GPA. Patients with a history of use of either CYC or RTX before the study were excluded from our study.
Ethical Considerations
This study was done in compliance with the WMA Declaration of Helsinki for ethical standards of medical research. All included patients signed an informed consent. This study was also accredited and registered by the ethical committee at Isfahan University of Medical Sciences in September 2020 under the registration code IR.MUI.MED.REC.1399.518.
Exposure
Treatments were prescribed by the treating physician independent of the physician who assessed outcomes. RTX was prescribed in four 500-mg doses in weekly intervals in line with corticosteroid pulses as induction therapy followed by 1 g maintenance doses every 6 months for maintenance. In the control group, pulses of CYC and prednisolone were administered for induction followed by azathioprine for maintenance. Both groups received prednisolone during maintenance initiated at 1 mg kg−1 day−1 and gradually tapered to 5 mg day−1. Adherence to treatment was ensured by a rheumatology fellowship assistant by tracking the patients’ visits (at three points: month 0, month 6, and month 12).
Outcomes
Primary outcomes were reported as major complications of the disease, including premature death, relapse, pulmonary failure, and renal involvement. The Birmingham Vasculitis Activity Score (BVAS) index was used to indicate disease activity before and after the induction therapy.16
We also assessed drug-related complications: (1) side effects of injectable RTX (severe mucocutaneous reactions, infection, and autoimmunity); (2) side effects of CYC (leukopenia, thrombocytopenia, bone marrow suppression, and severe nausea and vomiting). Missing case data were completed via telephone calls with the patient or their family by a senior rheumatology fellowship assistant.
Statistical analysis
The basis of the analysis in our study was the comparison of the incidence of complications across study groups reported. The CYC group was designated as the reference cohort (nonexposure group), and effect estimates for each cohort were calculated in relation to the reference cohort. We compared mean changes from baseline between the cohorts using ANOVA. The effect size was calculated as relative risk and number need to treat (NNT) with their respective confidence intervals. We adjusted for the effects of confounding using Cochran–Mantel–Haenszel and logistic regression statistics. A P-value of less than .01 was considered as significant. The data were analyzed using Statistical Package for the Social Sciences (SPSS) version 26 (IBM Corp.; Armonk, NY, USA).
Results
Baseline characteristics
We included 92 patients with GPA with the mean age of 44.7 (±14). Thirty-one (33.7%) patients suffered from comorbidities, and 19 patients were concomitantly being treated with other medications. All patients were followed-up for at least 6 months. The study cohorts did not defer at baseline in their demographic and disease-related characteristics (Table 1).
Table 1.
Patient Characteristics in Study Cohorts
| Patient Characteristics | Rituximab | Cyclophosphamide |
|---|---|---|
| Age (years) | 43 (13) | 47 (15) |
| Mean (SD) | ||
| Sex Number (percent) | ||
| Male | 19 (48%) | 27 (51%) |
| Female | 20 (52%) | 26 (49%) |
| Baseline BVAS, mean (SD) | 7.77 (1.01) | 7.21 (1.63) |
| Disease duration (years), mean (SD) | 3.46 (2.1) | 3.75 (1.98) |
| Comorbidities, N (%) | 10 (37%) a | 9 (31%) b |
| Serum Cr | 1.6 (1.9) | 2.4 (3.3) |
| ESR | 62.5 (32.3) | 72.9 (35.2) |
| CRP | 52.3 (38.4) | 61.1 (35.6) |
| Total, N (%) | 39 (42.4%) | 53 (57.6%) |
aDiabetes: three patients; hypertension: three patients; hypothyroidism: two patients; peptic ulcer: one patient; migraine: one patient.
bHypertension: two patients; diabetes: three patients; hypothyroidism: one patient; disk herniation: one patient; coronary artery disease: two patients.
BVAS, Birmingham Vasculitis Activity Score; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SD, standard deviation.
Exposure
Fifty-three (57.6%) patients received CYC, whereas 39 (43.4%) received RTX. The mean duration of treatment was 3.6 (±2) years.
Disease activity
The majority of the patients (70%) achieved and remained in remission, while 20.7% had a relapse and 8.7% died during the course of the study period. The rate of death and relapse did not differ between CYC and RTX groups (P value = .45).
Most patients (82.6%) did not develop disease-related complications. The rate of complications was lower among patients in the RTX group (4/39) compared to the CYC group (12/53) although this difference was not statistically significant (P value = .12). The relative risk and confidence intervals for each of the outcomes are presented in Table 2.
Table 2.
Effect Estimates for Main Study Outcomes
| Outcome | Relative Risk (95% CI) | NNT (95% CI) | P | |
|---|---|---|---|---|
| Disease severity | Death | 0.45 (0.09-2.1) | 16.14 (18.5 (harm) to ∞ to 5.6 (benefit)) | .3 |
| Relapse | 0.8 (0.3-1.8) | 21.3 (8.3 (harm) to ∞ to 4.7 (benefit) | .6 | |
| Pulmonary involvement a | 0.46 (0.01-11) | 66 (22-13) | .6 | |
| Renal involvement (CKD) b | 0.22 (0.02-1.8) | 11.4 (48.547 (harm) to ∞ to 5.109 (benefit)) | .1 | |
| Renal involvement (ESRD) c | 0.54 (0.11-2.6) | 23 (15.100 (harm) to ∞ to 6.564 (benefit)) | .4 | |
| Peripheral neuropathyd | 1.3 (0.08-21) | 147 | .8 | |
| Overall rate of complications | 0.45 (0.1-1.2) | 8 (32.442 (harm) to ∞ to 3.590 (benefit)) | .1 | |
| Medication adverse effects | 0.67 (0.06-7.2) | 82 (16.312 (harm) to ∞ to 11.696 (benefit)) | .7 | |
Including lung parenchymal nodules, interstitial lung disease, and alveolar hemorrhage.
Any type of renal involvement without end-stage renal disease and including glomerulonephritis, proteinuria, or hematuria with or without impaired renal function.
Severely impaired renal function needing dialysis.
Neuropathy resulting in glove and/or stocking distribution of sensory loss.
CKD, chronic kidney disease; CI, confidence interval; ESRD, end-stage renal disease; NNT, number need to treat.
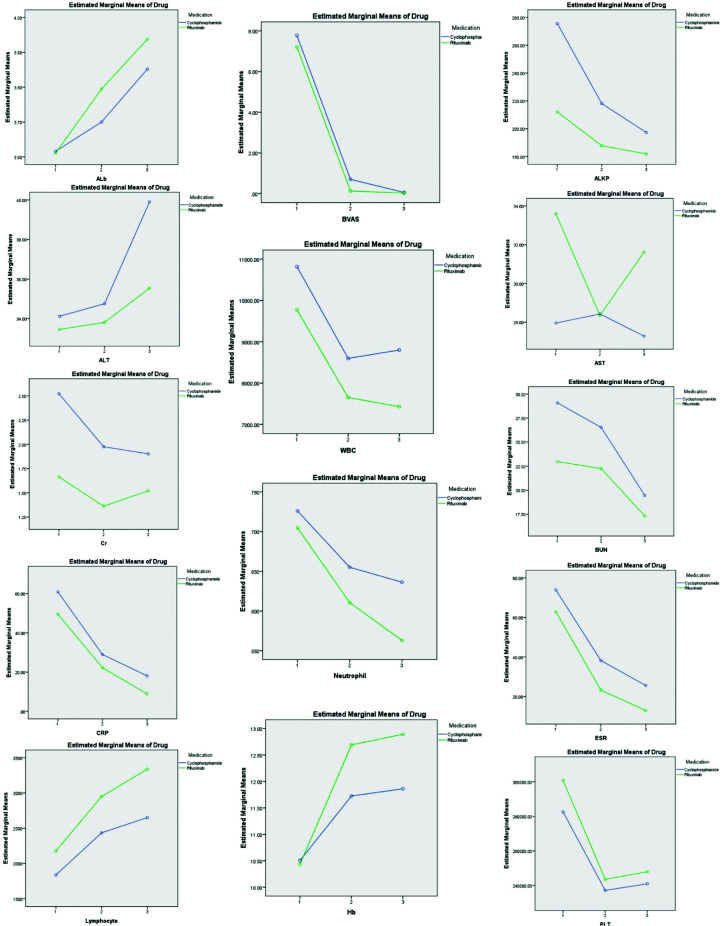
The BVAS decreased significantly in both groups during the course of follow-up. However, the change was not significantly different between medication exposure groups (P value = .19). There was a significant change from baseline for most of the measured laboratory markers during the course of the study with the exception of Aspartate aminotransferase (AST), Alanine transaminase (ALT), and serum Cr, which did not show a consistent trend throughout the follow-up period irrespective of the exposure (Figure 1 and Table 3). The trend of changes in mean laboratory values during the follow-up was not significantly different based on medication exposure groups.
Figure 1.
Laboratory markers trends during the study. Similar trends were seen in all laboratory markers between the study cohorts (i.e., the differences were insignificant).
Table 3.
The F Statistics for Laboratory Markers of Disease Activity
| Test | Greenhouse–Geisser (F Statistics) | P |
|---|---|---|
| WBC | 0.14 | .84 |
| Neutrophil | 1.39 | .25 |
| Hb | 2.43 | .10 |
| Plt | 0.18 | .805 |
| Lymphocyte | 0.81 | .444 |
| Erythrocyte Sedimentation Rate (ESR) | 0.151 | .821 |
| CRP | 0.150 | .844 |
| Blood Urea Nitrogen (BUN) | 0.322 | .707 |
| Cr | 0.782 | .421 |
| AST | 0.980 | .367 |
| ALT | 0.160 | .792 |
| Alkaline phosphatase (ALKP) | 0.64 | .492 |
| Albumin | 0.47 | .590 |
AST, aspartate aminotransferase; ALT, alanine transaminase; ALKP, alkaline phosphatase; BUN, blood urea nitrogen; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; Plt, platelet count; WBC, white blood cell.
Drug-related adverse events
Only two (3.3%) patients showed drug-related adverse effects. The medication type did not affect the rate of adverse-events (P value = .7) (Table 2).
Discussion
Our study did not show any difference in terms of clinical and para-clinical outcomes between RTX and CYC as induction therapy in patients with GPA.
The Rituximab versus cyclophosphamide in ANCA-associated vasculitis (RITUXVAS) trial that included 44 patients with newly diagnosed ANCA-associated vasculitis with renal involvement to remission induction regimens involving either RTX or CYC reported the same rate of clinical improvement and adverse events across the trial groups.17 The 2-year follow-up report of the same trial did not show any significant difference in the composite outcome of death, end-stage renal disease, and relapse between the trial groups.18 These results are consistent with the results of our study in that RTX and CYC perform similarly to induce remission in GPA and have been shown to cause the same rates of adverse events.17,18
Some researchers proposed the use of antibody phenotypes instead of clinical diagnoses for the choice of appropriate immunosuppressant in patients with ANCA-associated vasculitis, and that patients with PR3-ANCA (which may be associated with more severe disease) may respond better to RTX compared to CYC.19 Another study postulated that the expression of Fc receptor-like 5 may affect the clinical responsiveness to RTX irrespective of clinical and serological morphologies in ANCA-associated vasculitis.20 In our study that included only PR3-positive patients, five out of 39 patients in the RTX group compared to 12 out of 53 patients in the CYC group developed disease-related complications during follow-up. Although the difference was not statistically significant in our study, these factors may have been an underlying reason for the better response rate among patients who received RTX.
Our study presented 4-year outcomes of CYC compared to RTX for remission induction in patients with GPA, which is unique in its long follow-up. However, the nonrandomized assignment of patients to medication groups and lack of observation over adherence to exposure are some of the limitations associated with the retrospective observational design. The outcome measurement and recording may have been subject to bias due to interobserver variability and open label nature of prescriptions in an observational study. Moreover, considering the scarcity of GPA in prevalence, our study is very imprecise due to limited sample size.
We did not include c-ANCA-negative GPA patients in our study. This limits the generalizability of our results. Moreover, assessing the chronic kidney disease (CKD) patients’ clinical response to induction therapy in particular was not part of our study design, contrary to the 2015 study by Geetha et al.21 that reported similar responses to induction therapy with RTX compared to CYC among patients with renal involvement.
In this study, remission induction with RTX and CYC resulted in similar response rates among patients with GPA. Further studies with interventional design and randomization are needed to find predictive factors of response to either of the drugs and elucidate the application of our findings in clinical practice.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.518) (Approval Date: September 28, 2020).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.S.; Design - M.T.C., M.S.; Supervision - M.S.; Data Collection and/or Processing - M.T.C.; Analysis and/or Interpretation - Z.F.; Literature Review - M.T.C., M.S.; Writing - M.T.C.
Declaration of Interests: The authors have no conflicts of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Cotch MF, Hoffman GS, Yerg DE, Kaufman GI, Targonski P, Kaslow RA. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum . 1996;39:(1):87–92.. 10.1002/art.1780390112 [DOI] [PubMed] [Google Scholar]
- 2. Stone JH, Merkel PA, Spiera R.et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med . 2010;363:(3):221–232.. 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calich AL, Puechal X, Pugnet G.et al. Rituximab for induction and maintenance therapy in granulomatosis with polyangiitis (Wegener’s). Results of a single-center cohort study on 66 patients. J Autoimmun . 2014;50:135–141.. 10.1016/j.jaut.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 4. Jennette JC, Falk RJ, Bacon PA.et al. 2012 Revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum . 2013;65:(1):1–11.. 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 5. You C, Ma L, Lasave AF, Foster CS. Rituximab induction and maintenance treatment in patients with scleritis and granulomatosis with polyangiitis (Wegener’s). Ocul Immunol Inflamm . 2018;26:(8):1166–1173.. 10.1080/09273948.2017.1327602 [DOI] [PubMed] [Google Scholar]
- 6. Yates M, Watts RA, Bajema IM.et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis . 2016;75:(9):1583–1594.. 10.1136/annrheumdis-2016-209133 [DOI] [PubMed] [Google Scholar]
- 7. Martin F, Chan AC. Pathogenic roles of B cells in human autoimmunity. Immunity . 2004;20:(5):517–527.. 10.1016/S1074-7613(04)00112-8 [DOI] [PubMed] [Google Scholar]
- 8. Stevenson HC, Fauci AS. Activation of human B lymphocytes. XII. Differential effects of in vitro cyclophosphamide on human lymphocyte subpopulations involved in B-cell activation. Immunology . 1980;39:(3):391–397.. [PMC free article] [PubMed] [Google Scholar]
- 9. Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol . 1999;103:(5 Pt 1):885–894.. 10.1016/S0091-6749(99)70434-3 [DOI] [PubMed] [Google Scholar]
- 10. Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol . 1982;128:(6):2453–2457.. [PubMed] [Google Scholar]
- 11. Hoffman GS, Kerr GS, Leavitt RY.et al. Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med . 1992;116:(6):488–498.. 10.7326/0003-4819-116-6-488 [DOI] [PubMed] [Google Scholar]
- 12. Hassan RI, Gaffo AL. Rituximab in ANCA-associated vasculitis. Curr Rheumatol Rep . 2017;19:(2):6. 10.1007/s11926-017-0632-1 [DOI] [PubMed] [Google Scholar]
- 13. Benucci M, Manfredi M, Puttini PS, Atzeni F. Predictive factors of response to rituximab therapy in rheumatoid arthritis: What do we know today?. Autoimmun Rev . 2010;9:(12):801–803.. 10.1016/j.autrev.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 14. Quartuccio L, Fabris M, Salvin S, Maset M, De Marchi G, De Vita S. Controversies on rituximab therapy in Sjogren syndrome-associated lymphoproliferation. Int J Rheumatol . 2009;2009:1–8.. 10.1155/2009/424935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum . 2001;44:(12):2836–2840.. [DOI] [PubMed] [Google Scholar]
- 16. Kermani TA, Cuthbertson D, Carette S.et al. The Birmingham vasculitis activity score as a measure of disease activity in patients with giant cell arteritis. J Rheumatol . 2016;43:(6):1078–1084.. 10.3899/jrheum.151063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones RB, Cohen Tervaert JW, Hauser T.et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med . 2010;363:(3):211–220.. 10.1056/NEJMoa0909169 [DOI] [PubMed] [Google Scholar]
- 18. Jones RB, Furuta S, Tervaert JW.et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis . 2015;74:(6):1178–1182.. 10.1136/annrheumdis-2014-206404 [DOI] [PubMed] [Google Scholar]
- 19. Unizony S, Villarreal M, Miloslavsky EM.et al. Clinical outcomes of treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis based on ANCA type. Ann Rheum Dis . 2016;75:(6):1166–1169.. 10.1136/annrheumdis-2015-208073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Owczarczyk K, Cascino MD, Holweg C.et al. Fc receptor-like 5 and anti-CD20 treatment response in granulomatosis with polyangiitis and microscopic polyangiitis. JCI Insight . 2020;5:(18):e136180. 10.1172/jci.insight.136180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geetha D, Specks U, Stone JH.et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol . 2015;26:(4):976–985.. 10.1681/ASN.2014010046 [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Content of this journal is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.