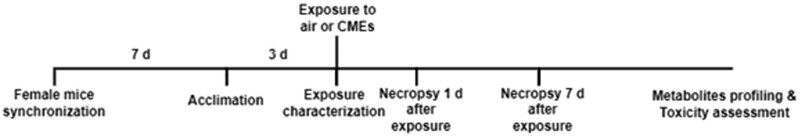
Figure 1.
Timeline of animal study. After arrival, 13-week-old mice were randomly placed in cages. Female mice estrous cycles were synchronized and all mice were acclimated to exposure chamber 3 days prior to exposures. During exposures, photoionization detector (PID) and gas chromatography-mass spectrometry (GC-MS) were used to monitor and characterize CMEs. Half of the mice were necropsied 1 day after exposure to determine acute responses. The other half of the mice was necropsied at 7-day postexposure to examine recovery. Assessment of serum styrene-specific metabolites was performed to determine exposure whereas serum metabolite and lipid profiling were utilized to identify biomarkers of exposure and biological response. Assessment of toxicity endpoints included alterations in bronchoalveolar lavage fluid (BALF) markers of inflammation and lung injury (immune cell influx, total protein concentration, cytokine/chemokine levels), gene expression of inflammatory, oxidative stress, and injury markers in the unlavaged lung tissue, and gene expression of inflammation and oxidative stress markers in brain tissue.