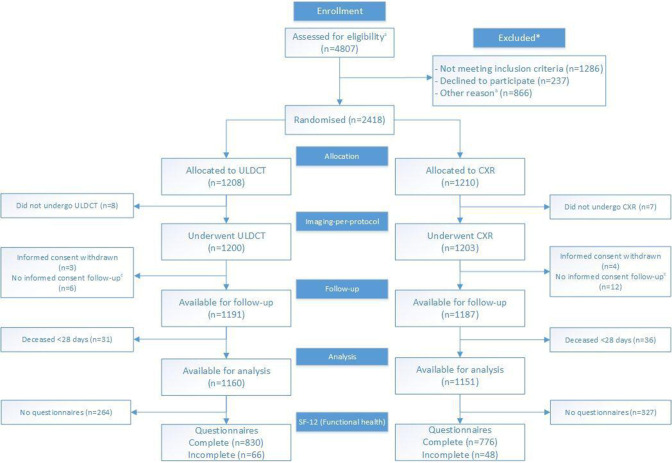
Figure 1.
Trial profile. CXR, chest X-ray; SF-12, Short Form 12; ULDCT, ultra-low-dose chest-CT. a Due to privacy regulations the total number of patients assessed for eligibility, and the total number of patients excluded for randomisation are incomplete. These numbers are composed of complete data (Amsterdam UMC, location AMC) and data from a random sample of non-included patients (Spaarne Gasthuis). Specified in online supplemental table 1. b Specified in online supplemental table 1. c Short informed consent form signed at the emergency department as patient was too ill for full consent, giving permission to use imaging information for study purposes. No full informed consent form, giving permission for collection of follow-up information was signed.