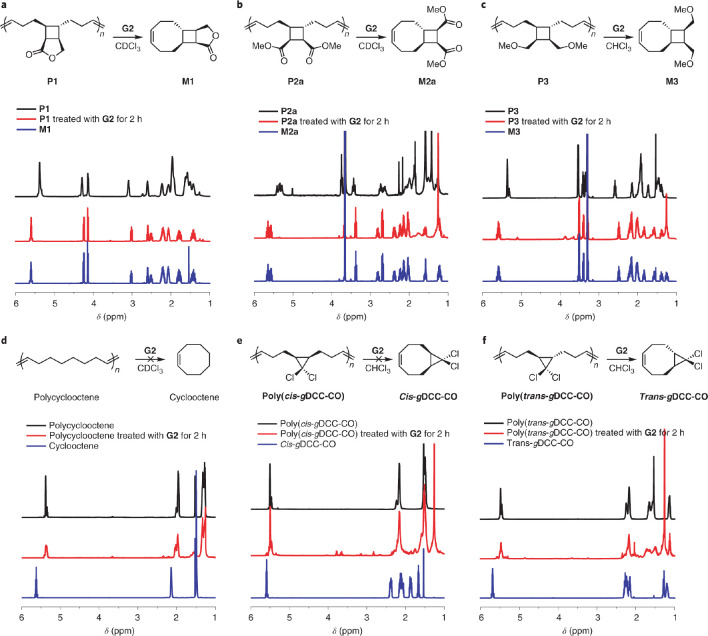
Figure 12.
(a–f) 1H NMR spectra for P1 (a), P2a (b), P3 (c), polycyclooctene (d), poly(cis-gDCC–CO) (e), and poly(trans-gDCC–CO) (f) before (in black) and after (in red) 2 h of heating the polymer solution (solvent, chloroform or deuterated chloroform; [olefin] = 25 mM) at 50 °C in the presence of Grubbs’s second-generation catalyst (G2). The 1H NMR spectra of the corresponding monomers are shown (in blue) as references. The red spectra (polymer treated with G2) and blue spectra (monomer) are nearly identical for tCBCO polymers, indicating complete depolymerization (a–c); however, the spectra are distinct for polycyclooctene, poly(cis-gDCC–CO), and poly(trans-gDCC–CO), suggesting no depolymerization occurred (d–f). Figure reproduced from ref (80) with permission from Springer Nature. Copyright 2021, Springer Nature.