Abstract
Opioid use and prescribing have become a subject of increasing focus and scrutiny. The ongoing “opioid epidemic” in North America has further increased interest in this area. In patients presenting for surgery, the prescribing of opioids during and following admission to hospital is commonplace and has been identified as a potential contributor to the growing opioid problem in North America. This review aims to present the timeline of the “opioid epidemic” as well as to introduce the concept of a “Transitional Pain Service”. The Transitional Pain Service is a multidisciplinary service originating at Toronto General Hospital that employs a multi-faceted approach to monitoring opioid use after discharge from surgery, and aims to safely wean patients from opioids while maintaining effective pain management. This approach and its results will be discussed in this review.
Keywords: Transitional Pain Service, opioids, analgesia, chronic post-surgical pain, chronic pain, opioid weaning
The use of opioids to treat chronic non-cancer pain has increased dramatically in the last decade, particularly in North America [1]. Simultaneously, this increase in prescribing practice has been correlated with an almost four-fold increase in opioid- related deaths (i.e. overdosing) [2]. In Canada, apparent opioid-related overdose deaths have claimed the lives of over 11,500 people between 2016 and 2018 [3]. Moreover, postoperative chronic pain is common (experienced by as many as one third of surgical patients) and often dealt with by prescribing opioids inappropriately without long-term follow-up [4]. Despite recent efforts to control the prescription of opioids and the introduction of abuse-deterrent opioid formulations, the epidemic is still yet to be fully targeted with a multi-factorial approach [5]. This review will describe a brief history of the opioid epidemic, its current state, and provide a detailed description of the concept of a Transitional Pain Service (TPS). A TPS is a novel program which aims to effectively manage acute pain post-operatively, facilitate opioid weaning, reduce the development of chronic pain disability, and potentially help to flatten the worrisome trajectory of opioid-related deaths associated with excessive opioid prescribing.
HISTORY OF THE OPIOID EPIDEMIC
In order to give some context into how the opioid epidemic arose, it is imperative to examine patterns beginning in the 1980s. At that time, pharmaceutical companies marketed the use of opioids to treat pain and assured clinicians that the addiction profile of these analgesic agents was low [6]. Within a few years, the same companies promoted these drugs for use in long-term non-cancer pain, despite the lack of good evidence to ensure efficacy in the treatment of chronic pain in this population [7]. This misrepresentation on the part of the pharmaceutical companies sparked the first of three major waves of increased opioid prescribing. The number of opioid prescriptions increased among primary care clinics and hospitals [2], and with that an increased amount of opioids available for diversion – unlawful channeling of regulated pharmaceuticals from legal sources to the illicit marketplace [8] – took place [2]. Pharmaceutical companies responded to the public outcry by developing a sustained-release opioid formulation know as oxycodone (branded as OxyContin). This new formulation required fewer administrations daily, compared to taking pain medications every 2–4 hours as previously [9]. The Food and Drug Administration concluded that the sustained-release formulation of oxycodone would prevent abusive drug behavior due to its delay in reward which is necessary to reinforce addictive behavior [9]. This resulted in a false sense of security among prescribers, leading to an increase in OxyContin prescribing from 1997 to 2002 [2]. However, users circumvented the sustained release by crushing the tablets and delivering the medication intranasally or intravenously to deliver a potent dose of the drug. This led to increased rates of opioid oversedation [5] and eventually more opioid-related deaths. In light of these events, Purdue Pharma pleaded guilty to understating the risk of addiction with respect to OxyContin [2]. Effective March 1, 2012 Purdue Pharma pulled OxyContin from the market and introduced OxyNeo – an oxycodone formulation with a tamper-resistant tablet to reduce abuse [10].
The second wave began around 2010, as the increased media attention to the crisis led to an inability to access prescription opioids. This inevitably led to the rise in popularity of a cheap, widely available option – heroin [11]. Corresponding with the rise in heroin use, this marked the peak of the annual prescribing rate and average daily morphine equivalents (MEQ) in a decade or so [12]. In 2015, nearly a quarter of drug overdose deaths were due to heroin making up over 15,000 deaths that year in the U.S. [2]. Despite best efforts from clinicians, government agencies, and public health workers, the problem was still rapidly growing, making any solution proposed seem unfeasible.
Finally, the last resurgence was seen in 2013 (Figure 1) and was likely due to synthetic analog derivatives of fentanyl such as acetylfentanyl, butyrylfentanyl, carfentanil and W-18 [13]. The sharpest increase in drug-related deaths due to fentanyl, however, occurred in 2016, accounting for 20,000 deaths in the U.S. [14]. A substantial amount of the fentanyl consumed in Canada has been noted to originate from overseas through illicit trade as opposed to prescription for chronic pain [15]. These astounding statistics provided the framework for motivation and need for hospital level changes through better education and more secure storage of these drugs. The Nova Scotian, British Columbian and Ontario’s government responded by utilizing a Narcotics Monitoring System to ensure adequate monitoring of prescription opioids [16].
FIGURE 1.
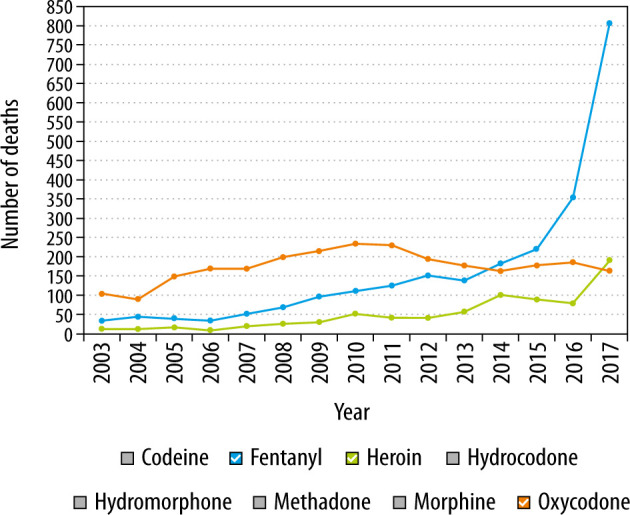
Opioid-related deaths in Ontario, Canada, 2003–2017
CHRONIC POST-SURGICAL PAIN
Expectedly, when a patient undergoes a surgical procedure, some level of pain is experienced postoperatively, and the severity and duration vary from patient to patient [17]. When this pain develops after surgery and persists for at least 2 months, it is referred to as chronic post-surgical pain (CPSP) [18]. Opioid prescribing rates have increased as a result of an increased demand for pain management [19]. Additionally, in a recent review of US insurance data, surgical patients’ mean total oral MEQ per prescription has almost doubled from 240 mg in 2010 to 403 mg in 2016 [19].
The heterogeneity in the patient pain experience relies on a multitude of factors related to the patient, type of procedure, and postoperative recovery environment. The primary predictor of CPSP is a prior pain history, while poorly controlled acute postoperative pain is important to consider, as well as other patient-related factors including female gender and younger age [17, 20–22]. Surgical risk factors for CPSP such as the surgical approach (open vs. laparoscopic) and duration of surgery must be considered. Finally, psychosocial constructs that are often left unrecognized are increased preoperative anxiety, poor social supports, and engaging in catastrophizing behavior – predicting a negative pain outcome related to the surgery [17].
The literature suggests that patients are currently complaining more of pain. The PAIN OUT study demonstrated that patients in American society complain more of similarly painful experiences versus their European and international counterparts [23]. After similar orthopedic procedures, American patients rated higher “worst pain” scores than the international cohort (made up of European patients and patients outside of Europe). Interestingly, the difference in pain experiences was independent of typical risk factors such as female gender, younger age, high body mass index (BMI), chronic pain, and opioid use before surgery [23]. In a comparison of American and Dutch patients following hip and ankle surgery, American patients required post-discharge opioid prescriptions in 77% of cases compared to none among Dutch patients, reflecting as much a prescribing cultural difference as well as a philosophical pain management difference [24].
ISSUES OF POSTOPERATIVE OPIOID PRESCRIBING
Data demonstrate that a significant number of patients experience CPSP at three months postoperatively, particularly patients who have undergone thoracic and breast surgery [25]. Opioids are often prescribed to address this in the acute postoperative setting; however, once re-prescribed, this is typically done without a long-term plan, or with a plan to eventually wean these medications. Alam and colleagues noted that patients receiving an opioid prescription within the first week of surgery were 44% more likely to be taking them one year later compared to those who did not receive the same prescription [26]. Opioids were found to increase the risk of road trauma by 21% even at low doses (20–49 MEQ), while patients taking moderate to high doses of opioids were at 29–42% increased odds of motor vehicle accidents [27]. There are also data to suggest that opioid-related deaths have increased as a result of opioid prescribing, over half of which have been deemed unintentional [28]. Finally, opioid use was associated with a higher prevalence of constipation and reduced quality of life in patients with an opioid dependence [29].
Patients are ultimately left with inappropriately managed chronic pain and almost half of these patients continue to use opioids as more of a “band-aid” solution. The issue becomes more complex as these patients seek care from their primary care physicians and surgeons. These physicians often struggle with complex chronic pain management and lack the expertise to wean patients off opioids [25]. With CPSP estimated to cost U.S. $41,000–43,000 annually per patient, it is important that there are programs and processes in place to address this issue [25, 30]. Mayo Clinic recently developed guidelines to aid practitioners in prescribing opioids for adults upon discharge after common surgical procedures [31]. Educational interventions focused on educating surgeons on using NSAIDs and acetaminophen before prescribing opioids led to a 53% decrease in the amount of opioids prescribed to general surgery patients [32].
Although some success has been seen with the implementation of prescribing guidelines and educational interventions, new programs need to be implemented to address this systemic issue. This led to the introduction of a Toronto General Hospital based TPS with a focus on patients at high risk of developing chronic pain and patients with complex pain management and opioid weaning.
CONCEPT OF A TRANSITIONAL PAIN SERVICE
The TPS was created to effectively manage pa-tients’ perioperative pain long-term if needed, maintain function, reduce opioid consumption, and monitor the efficacy of these interventions. Approximately 7% of all major surgical interventions result in a pain disability problem one year later, and this has implications for both CPSP and long-term opioid use with respect to patient recovery. The TPS was launched in 2014 and it is the first program of its kind to target CPSP at three stages: 1) preoperatively, 2) postoperatively in the hospital setting, 3) postoperatively in the outpatient setting for up to 6 months after surgery [30, 33]. The services offered by the TPS are broken down into three categories: 1) Introduction and optimization of multimodal analgesia to both improve pain management and facilitate weaning from opioids; 2) non-pharmacologic interventions including physiotherapy and acupuncture; 3) psychological interventions by a trained pain psychology team around an acceptance and commitment therapy (ACT) model [30]. The focus of ACT is pain education and pain coping by fostering commitment in valued life activities, while encouraging acceptance and mindfulness of the distressing pain experience [34, 35].
The TPS treats two types of patients: opioid-naïve patients without a preexisting pain condition (5–10% risk for the development of moderate to severe CPSP) and patients presenting with a preexisting pain condition or on preoperative opioid medications (population ranges from 10–20%, 12.5% among patients at Toronto General Hospital) [30, 36]. This is managed through an interdisciplinary team which is composed of anesthesiologists, acute pain nurse practitioners, clinical psychologists, palliative care specialists, an exercise physiologist, and a patient-care coordinator [33]. With the goal of minimizing risk of developing CPSP before surgery and long-term opioid use postoperatively, the TPS identifies high-risk pain patients during the pre-admission clinic visit [36]. Patients that may have been missed during their pre-admission visit could still be referred to the service if they met any criteria including pre-surgical chronic pain, history of drug abuse, severe post-surgical pain, high post-surgical opioid consumption (> 90 MEQ/day), and emotional distress [30]. These patients are followed up as inpatients to avoid delays in discharge due to uncontrolled pain. Once discharged, early follow-up is arranged (within 1–2 weeks), such that they continue to be seen on an outpatient basis with the eventual goal of transitioning them back to their primary care physician.
EARLY SUCCESS OF THE TRANSITIONAL PAIN SERVICE
The TPS program based out of the Toronto Gene-ral Hospital has been operational for five years and there have been a number of notable successes. Between May 2014 and July 2017, the TPS analyzed 304 surgical patients after discharge from hospital. Of these, 251 had full data available for review and analysis. These patients were classified into two groups: opioid-naïve (0 MEQ/day) (45%) or opioid-experienced (> 0 MEQ/day) (55%).
Among opioid naïve patients, MEQ consumed were decreased from 106.7 post-surgery to 37.3 at the final TPS visit (6.6 months after surgery), translating to a 65% decrease [33]. Within the opioid-experienced group, 140.5 MEQ at discharge were decreased to 78.3 at the final TPS visit (5.2 months after surgery), resulting in a 44% decrease [33]. More importantly, among opioid-naïve patients, 44.5% of patients were no longer taking an opioid at 6 months after discharge, and 25.6% of opioid-experienced patients were completely weaned off opioids at 6 months. Among those still taking opioids, 45.5% and 55.4% of opioid-naïve and opioid-experienced patients saw some level of reduction in opioid consumption, respectively [33].
The above data demonstrate that the TPS has been effective in safely weaning patients from opioids in the postoperative period, in both opioidnaïve and experienced patients. At the same time, TPS involvement in the post-discharge period has also led to reductions in reported pain. In the thoracic surgery population, a cohort of patients followed by the TPS in the postoperative period reported a reduced level of pain and a faster trajectory towards “milder” pain as compared to a cohort without TPS involvement [37].
Within the TPS framework, importance is placed on the optimization of non-opioid adjuncts to enhance pain management and facilitate weaning of opioids. Such adjuncts include medical cannabis, which is rapidly gaining adoption in chronic pain management in Canada. For example, TPS was successfully able to facilitate opioid weaning in a post-liver transplant patient [38]. In this case, a patient requiring 30 mg of oral hydromorphone per day was successfully weaned to 6 mg per day through the introduction of medical cannabis as a pain adjunct. This not only produced significant reductions in opioid needs, but also improved self-reported functional status.
The success of the Toronto TPS experience has led to similar initiatives elsewhere. A similar model to the TPS has been adopted successfully in the United Kingdom [39], where non-postoperative patients with chronic pain participated in an interdisciplinary pain management program. This program involved psychologists, physiotherapists, nurses and physicians in a comprehensive program similar to that of the TPS. They also relied heavily on ACT-based theory. Overall, participants saw a reduction in the total oral morphine equivalents dose, as well as the number of medication classes used. This interdisciplinary approach to pain management has seen success, further establishing the need, importance, and feasibility of this model.
FUTURE PLANS FOR THE TRANSITIONAL PAIN SERVICE
The implementation of the TPS program, and its early success, have allowed for planning to expand the scope of the program, and increase accessibility. These plans include engaging community and family physicians to facilitate referral of non-Toronto General Hospital surgical patients to the program [30]. In addition, there are plans to develop rehabilitation programs incorporating yoga and mindfulness to improve physical and psychological wellbeing. With the increasing availability and adoption of mobile technology, the TPS has recently engaged patients in self-reporting of pain scores and functioning through mobile apps. This facilitates ongoing tracking and documentation, and allows patients to participate in their pain management more actively. Finally, the future of pain management must involve providing support and expertise to allow for the development of similar programs at other hospitals, allowing easier access for patients in need.
RECOMMENDATIONS FOR INITIATING SIMILAR SERVICE IN OTHER HOSPITALS
There are three main considerations when considering the implementation of a program similar to the TPS. These are institutional support, buy-in from surgeons, and comprehensive multidisciplinary involvement.
In terms of incentivizing institutional support, the potential cost and patient care benefits of a TPS program are clear. It is estimated that CPSP costs the system on average $41,000-43,000 per patient annually (U.S. System) [40]. Our own analysis based on the experience at Toronto General Hospital has revealed significant potential benefits within the Canadian healthcare system [25]. With 4000 patients receiving major surgery at the Toronto General Hospital annually, new cases and worsening cases of post-surgery chronic pain make up 5% and 12.5%, respectively, of all major procedures. With a gross underestimated annual cost of $5000 annually, it can be estimated that these patients access over $2.5 million of health care services annually from the system. These funds could be used to service other areas within patient pain management, or other areas of healthcare. Outside of the financial benefits, the availability of services such as the TPS can also alleviate strain on other departments within the hospital. This includes potentially reducing emergency department visits for patients needing assistance with pain management or prescriptions, which is a common cause for emergency department visits [41]. This allows the emergency departments and acute care clinics to focus on managing patients with acute or emergent medical issues. The combined potential to improve both efficiency in health care spending, as well as overall patient care, should provide sufficient incentive to most institutions.
The engagement of surgeons for support of a TPS program is equally important, and also straightforward. Pain management and medication prescribing is a common topic of both postoperative clinic visits with surgeons, and telephone calls to surgeon offices [42]. While issues of this nature are important, they often occupy a significant portion of busy surgical clinic visits and potentially distract from discussion of surgical or prognostic details. Additionally, some pain complaints are complex, and beyond the scope of what a surgeon may feel comfortable addressing. A TPS program would allow faster access to pain management specialists and allow surgeons to focus on important areas where they have more expertise.
Finally, the success of any program similar to TPS hinges upon the need for multidisciplinary involvement. The availability of non-pharmacologic interventions including physiotherapy and behavioral interventions grounded in Acceptance and Commitment Therapy are imperative to the improvement in pain management and reduction in opioids. Preliminary outcomes from the Toronto General Hospital showed that patients who participated in ACT saw a 17% mean pain score reduction vs 8% reduction for those patients who did not participate [43]. ACT patients also saw a significant reduction in pain interference scores – one’s pain experience leading to anxiety or depression – compared to those who did not participate in ACT.
CONCLUSIONS
In summary, this review provided a brief history of the opioid epidemic and defined the concept of chronic post-surgical pain. Furthermore, the rationale for the TPS was described (i.e. created in order to address patient’s pain management needs through a multidisciplinary model) and preliminary data regarding early successes were reported. Finally, recommendations were given to hospitals that are aiming to implement a similar pain management program, with insight into hurdles that may be faced and potential means to mitigate them.
ACKNOWLEDGEMENTS
Financial support and sponsorship
none.
Conflicts of interest
none.
References
- 1.Gomes T, Mamdani MM, Dhalla IA, Cornish S, Paterson JM, Juurlink DN. The burden of premature opioid-related mortality. Addiction 2014; 109: 1482-1488. doi: 10.1111/add.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones MR, Viswanath O, Peck J, Kaye AD, Gill JS, Simopoulos TT. A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther 2018; 7: 13-21. doi: 10.1007/s40122-018-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health Agency of Canada . National report: Apparent opioid-related deaths in Canada (January 2016 to December 2018). Ottawa: Special Advisory Committee on the Epidemic of Opioid Overdoses; 2019. [Google Scholar]
- 4.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg 2017; 152: 1066-1071. doi: 10.1001/jamasurg.2017.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker WC, Fiellin DA. Abuse-deterrent opioid formulations–putting the potential benefits into perspective. N Engl J Med 2017; 376: 2103-2105. doi: 10.1056/NEJMp1701553. [DOI] [PubMed] [Google Scholar]
- 6.Meldrum ML. The ongoing opioid prescription epidemic: historical context. Am J Public Health 2016; 106: 1365-1366. doi: 10.2105/AJPH.2016.303297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier R. A short history of pain management. CMAJ 2018; 190: E26-E7. doi: 10.1503/cmaj.109-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciardi JA, Surratt HL, Lugo Y, Cicero TJ. The diversion of prescription opioid analgesics. Law Enforc Exec Forum 2007; 7: 127-141. [PMC free article] [PubMed] [Google Scholar]
- 9.Cicero TJ, Ellis MS. The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues Clin Neurosci 2017; 19: 259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Division OPDP. Change in Funding Status of Oxycodone Controlled Release Tablet (Discontinuation of OxyContin and introduction of OxyNEO). Ottawa: 2012; p. 1-5. [Google Scholar]
- 11.Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers–United States, 2002-2004 and 2008-2010. Drug Alcohol Depend 2013; 132: 95-100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Alam A, Juurlink DN. The prescription opioid epidemic: an overview for anesthesiologists. Can J Anaesth 2016; 63: 61-68. doi: 10.1007/s12630-015-0520-y. [DOI] [PubMed] [Google Scholar]
- 13.Prekupec MP, Mansky PA, Baumann MH. Misuse of novel synthetic opioids: a deadly new trend. J Addict Med 2017; 11: 256-265. doi: 10.1097/adm.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciccarone D. Fentanyl in the US heroin supply: a rapidly changing risk environment. Int J Drug Policy 2017; 46: 107-111. doi: 10.1016/j.drugpo.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer B, Vojtila L, Rehm J. The ‘fentanyl epidemic’ in Canada–some cautionary observations focusing on opioid-related mortality. Prev Med 2018; 107: 109-113. doi: 10.1016/j.ypmed.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health and Long-Term Care . Narcotics monitoring system 2017, September 1. Available at: http://www.health.gov.on.ca/en/pro/programs/drugs/ons/monitoring_system.aspx.
- 17.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009; 9: 723-744. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 18.Merskey H, Bogduk N. International Association for the Study of Pain. Task Force on Taxonomy. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle: IASP Press; 1994, p. 222. [Google Scholar]
- 19.Larach DB, Waljee JF, Hu HM, et al. Patterns of initial opioid prescribing to opioid-naive patients. Ann Surg 2018. doi: 10.1097/SLA.0000000000002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014; 348: g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain 1996; 12: 50-55. [DOI] [PubMed] [Google Scholar]
- 22.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618-1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 23.Zaslansky R, Meissner W, Chapman CR. Pain after orthopaedic surgery: differences in patient reported outcomes in the United States vs internationally: an observational study from the PAIN OUT dataset. Br J Anaesth 2018; 120: 790-797. doi: 10.1016/j.bja.2017.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and The Netherlands. J Trauma 2009; 67: 160-164. doi: 10.1097/TA.0b013e31818c12ee. [DOI] [PubMed] [Google Scholar]
- 25.Huang A, Azam A, Segal S, et al. Chronic postsurgical pain and persistent opioid use following surgery: the need for a transitional pain service. Pain Manag 2016; 6: 435-443. doi: 10.2217/pmt-2016-0004. [DOI] [PubMed] [Google Scholar]
- 26.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 2012; 172: 425-430. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
- 27.Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA Intern Med 2013; 173: 196-201. doi: 10.1001/2013.jamainternmed.733. [DOI] [PubMed] [Google Scholar]
- 28.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ 2009; 181: 891-896. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lugoboni F, Mirijello A, Zamboni L, et al. High prevalence of constipation and reduced quality of life in opioid-dependent patients treated with opioid substitution treatments. Expert Opin Pharmacother 2016; 17: 2135-2141. doi: 10.1080/14656566.2016.1232391. [DOI] [PubMed] [Google Scholar]
- 30.Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res 2015; 8: 695-702. doi: 10.2147/JPR.S91924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiels CA, Ubl DS, Yost KJ, et al. Results of a prospective, multicenter initiative aimed at developing opioid-prescribing guidelines after surgery. Ann Surg 2018; 268: 457-468. doi: 10.1097/SLA.0000000000002919. [DOI] [PubMed] [Google Scholar]
- 32.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ Jr. An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg 2018; 267: 468-472. doi: 10.1097/SLA.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 33.Clarke H, Azargive S, Montbriand J, et al. Opioid weaning and pain management in postsurgical patients at the Toronto General Hospital Transitional Pain Service. Can J Pain 2018; 2: 236-247. doi: 10.1080/24740527.2018.1501669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011; 152: 533-542. doi: 10.1016/j.pain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and Commitment Therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain 2017; 33: 552-568. doi: 10.1097/AJP.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 36.Clarke H. Transitional Pain Medicine: novel pharmacological treatments for the management of moderate to severe postsurgical pain. Expert Rev Clin Pharmacol 2016; 9: 345-349. doi: 10.1586/17512433.2016.1129896. [DOI] [PubMed] [Google Scholar]
- 37.Clarke H, Poon M, Weinrib A, Katznelson R, Wentlandt K, Katz J. Preventive analgesia and novel strategies for the prevention of chronic post-surgical pain. Drugs 2015; 75: 339-351. doi: 10.1007/s40265-015-0365-2. [DOI] [PubMed] [Google Scholar]
- 38.Meng H, Hanlon JG, Katznelson R, Ghanekar A, McGilvray I, Clarke H. The prescription of medical cannabis by a transitional pain service to wean a patient with complex pain from opioid use following liver transplantation: a case report. Can J Anaesth 2016; 63: 307-310. doi: 10.1007/s12630-015-0525-6. [DOI] [PubMed] [Google Scholar]
- 39.Guildford BJ, Daly-Eichenhardt A, Hill B, Sanderson K, McCracken LM. Analgesic reduction during an interdisciplinary pain management programme: treatment effects and processes of change. Br J Pain 2018; 12: 72-86. doi: 10.1177/2049463717734016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons B, Schaefer C, Mann R, et al. Economic and humanistic burden of post-trauma and post-surgical neuropathic pain among adults in the United States. J Pain Res 2013; 6: 459-469. doi: 10.2147/JPR.S44939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulin PA, Nelli J, Tremblay S, et al. Chronic pain in the emergency department: a pilot mixed-methods cross-sectional study examining patient characteristics and reasons for presentations. Pain Res Manag 2016; 2016: 3092391. doi: 10.1155/2016/3092391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kharasch ED, Brunt LM. Perioperative opioids and public health. Anesthesiology 2016; 124: 960-965. doi: 10.1097/ALN.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 43.Azam MA, Weinrib AZ, Montbriand J, et al. Acceptance and commitment therapy to manage pain and opioid use after major surgery: preliminary outcomes from the Toronto General Hospital Transitional Pain Service. Can J Pain 2017; 1: 37-49. doi: 10.1080/24740527.2017.1325317. [DOI] [PMC free article] [PubMed] [Google Scholar]


