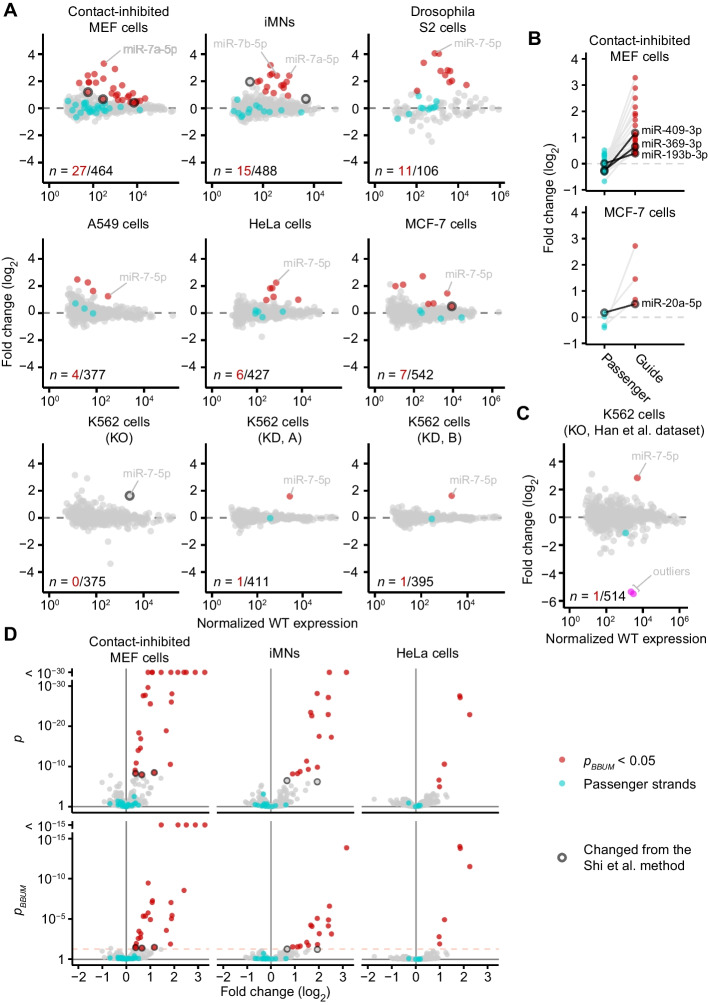
Fig. 3.
Identification of candidate ZSWIM8-sensitive miRNAs using the BBUM method. A Plots of log2 fold changes in miRNA levels observed upon loss of ZSWIM8 in all mammalian and fly cell lines examined in Shi et al. [10]. Two datasets were derived from CRISPRi knockdown (KD) of ZSWIM8 in K562 cells, using one of two different guide RNAs (A or B). All other datasets were derived from knockout (KO) of either ZSWIM8 or Dora, the Drosophila ZSWIM8 ortholog. Points for miRNAs that met the common pBBUM significance cutoff of 0.05 are in red, with n indicating the number passing this cutoff, shown as fraction of the total number of miRNAs and passenger strands quantified. Points for passenger strands of these miRNAs are in cyan, if the passenger strands were both annotated and observed above the expression threshold. Points for miRNAs with classifications differing from that of previous work [10] are outlined in black (n = 7). Points for miR-7-5p, a known TDMD substrate [16], are labeled. B Different effects of the loss of ZSWIM8 on miRNAs and their passenger strands. Fold changes in miRNA levels are shown for two datasets with newly significant miRNAs. Only miRNAs found significant by the BBUM method and whose passenger strands were quantified in the dataset are shown, with each miRNA paired with its passenger strand(s). Points for newly significant miRNAs are outlined and labeled. C Plot of fold changes in miRNA levels observed upon knockout of ZSWIM8 in K562 cells by Han et al. [11]. Points for two downregulated outliers (hsa-miR-221-3p, hsa-miR-222-3p) are shown in magenta. Otherwise this panel is as in A. D Volcano plots of raw and BBUM-FDR-adjusted p values. Colors are as in A. The significance cutoff at 0.05 is shown as orange dashed lines for pBBUM