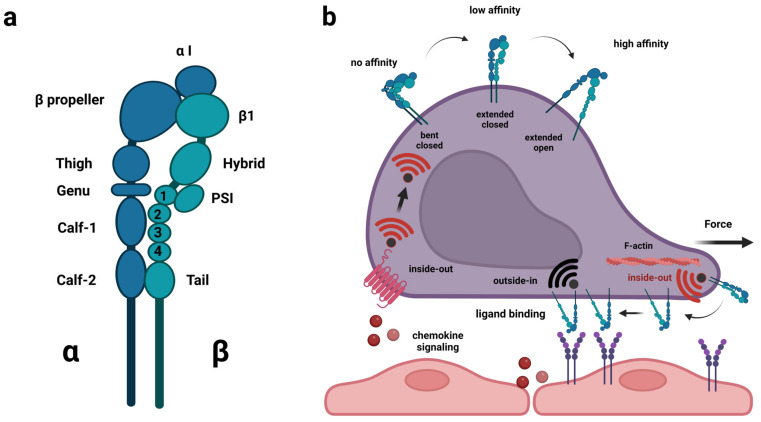
Figure 1.
Structure of integrin heterodimers and regulation of integrin affinity on the cell surface. (a) heterodimer composed of α and β subunit. Shown is LFA-1 (αLβ2). Integrin α-subunits expressed in leukocytes (αL, αM, αD and αX) as well as α1, α2, α10 and α11 contain the so-called insertion or interaction domain (αI), which plays a vital part in ligand-binding of these integrins. All other α-subunits bind ligands in conjunction with the β-subunit, i.e., the head piece. (b) integrin heterodimers shift between bent conformation with closed head piece (no affinity for ligand), extended conformation with closed head piece (intermediate affinity) and extended conformation with open head piece (high affinity). Chemokine signaling and forces from the inside (e.g., actin-dynamics) can cause a shift between the conformations (inside-out activation), whereas binding to ligands or external forces (e.g., blood flow) can cause outside-in activation. This figure was created with BioRender.com (accessed on 21 April 2023).