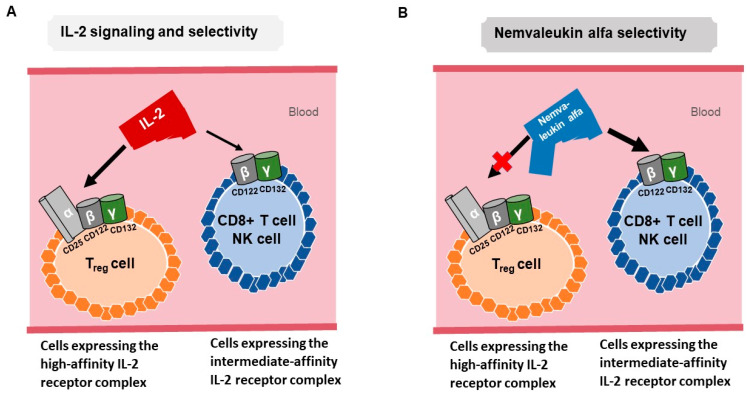
Figure 1.
(A) IL-2 binds to the high-affinity IL-2 receptor complex containing α, β, and γ subunits expressed on Treg and endothelial cells. This high-affinity selectivity can lead to the expansion of immunosuppressive Tregs and life-threatening toxicities. IL-2 also binds to the intermediate-affinity IL-2 receptor complex of β and γ subunits on CD8-positive cytotoxic T cells and NK cells. (B) Nemvaleukin alfa is an engineered IL-2-IL-2Rα fusion protein that selectively binds to the intermediate-affinity IL-2 receptor complex, where it preferentially activates CD8-positive cytotoxic T cells and NK cells rather than the expansion of Tregs, enhancing the anticancer immune effects mediated by CD8-positive T cells.