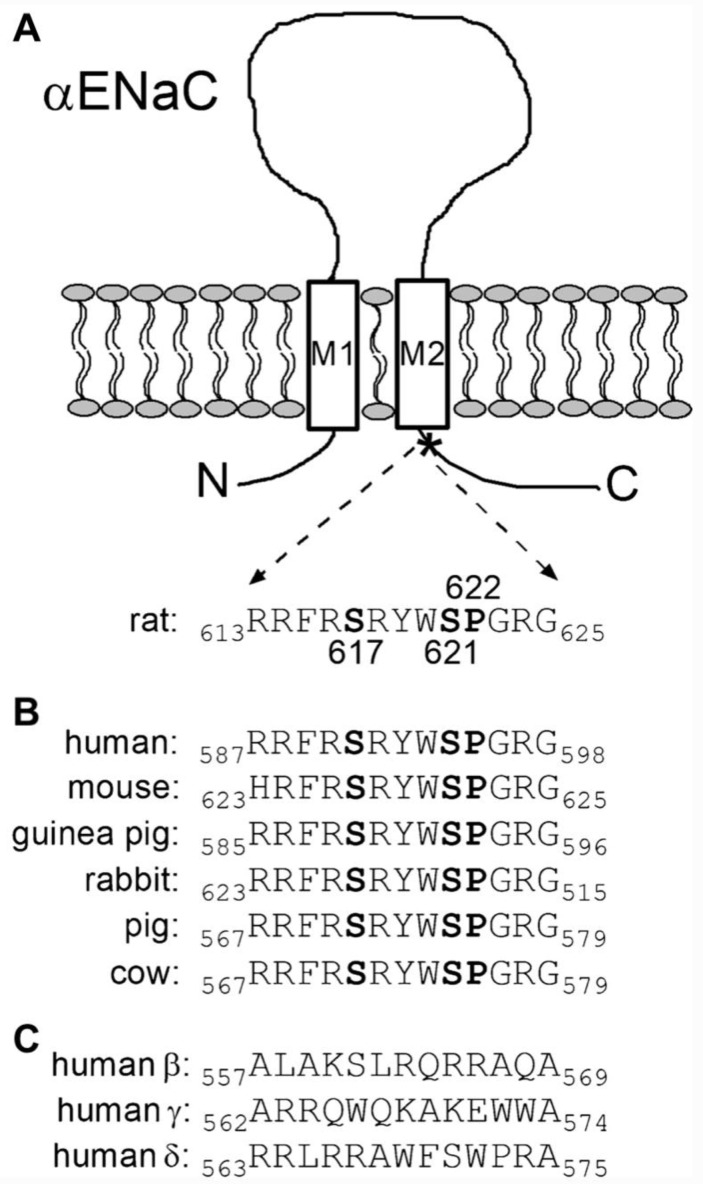
Figure 3.
Potential sites of phosphorylation in α-ENaC C-terminal region. Two serine residues and one proline residue are highly conserved in a C-terminal region of α-ENaC close to the second transmembrane domain. (A) Schematic representation of α-ENaC illustrating the extracellular loop, two transmembrane domains (M1 and M2) and intracellular N- and C-termini. The amino acid sequence of rat α-ENaC (residues 613–625) corresponds to the C-terminal region indicated by a star (*) and contains the serine residues 617 (S617) and 621 (S621) and the proline residue 622 (P622) highlighted in bold. (B) Amino acid sequence alignment of this highly conserved C-terminal region from several mammalian α-ENaC subunits. The residues homologous to S617, S621 and P622 in rat αENaC are highlighted in bold. (C) Amino acid sequence alignment of homologous C-terminal regions from human β-, γ- and δ-ENaC subunits, showing a lack of potential phosphorylation sites. Adapted from [110] (Figure 1); Copyright © The Author(s) 2022, corrected publication 2022; published by Springer Nature.