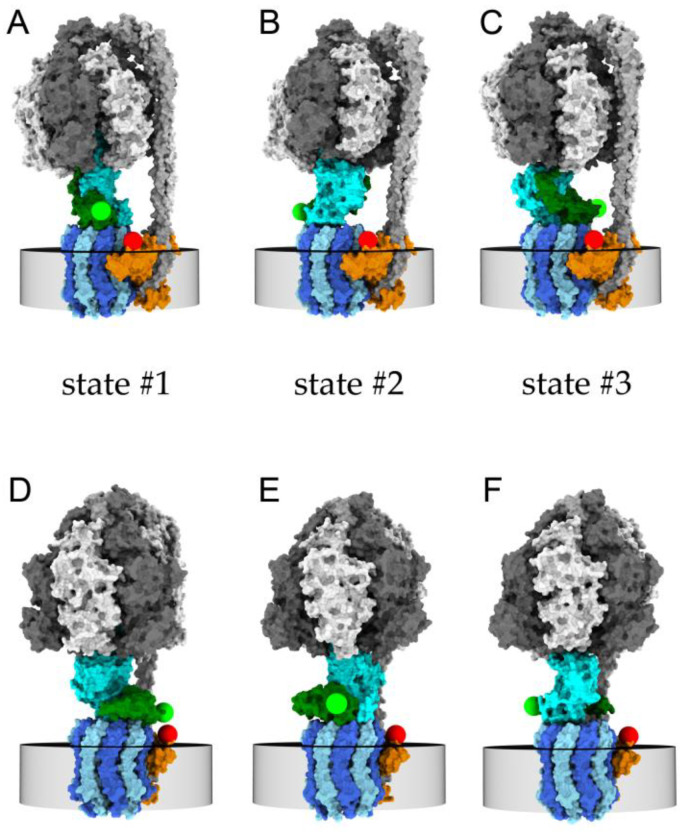
Figure 1.
Models of FRET-labeled FoF1-ATP synthase in the three rotary states of the γ- and ε-subunits with respect to the F1 subunits α3β3δ (white and dark gray) and Fo subunits b2 (light gray, peripheral stalk). (A–C) cryoEM structures in the presence of 10 mM ADP with subunits b2 oriented to the right side [35]. (D–F) cryoEM structures in the presence of 10 mM ATP with subunits b2 oriented to the back side [36]. Green spheres are positions of the S atom of cysteine εH56C on the ε-subunit (dark green) labeled with FRET donor Cy3B, red spheres are modeled positions of the S atom of cysteine a276C on the a-subunit (orange) labeled with FRET acceptor Alexa Fluor 647. The central rotary γ-subunit of the F1 domain is shown in cyan, and c-subunits of the Fo domain in dark blue and in light blue, embedded in the membrane (indicated by the gray disc). During ATP hydrolysis, the rotary states move sequentially in the order …→state #1 (D)→state #2 (E)→state #3 (F)→… for the counter-clockwise rotation direction when viewed from the bottom.