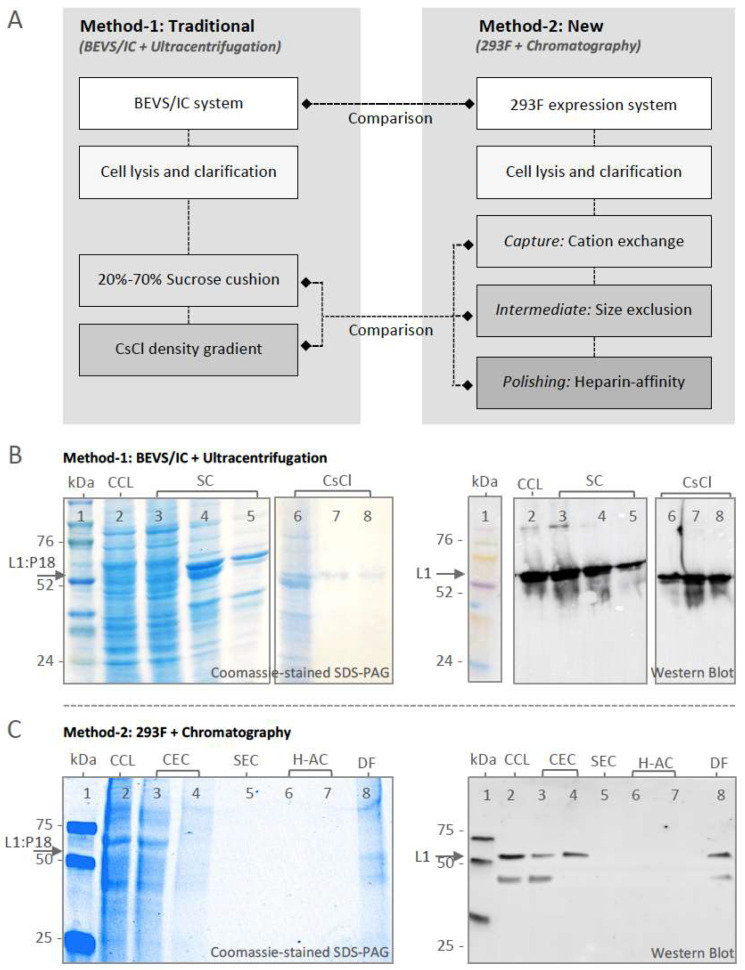
Figure 3.
Purification and characterization of L1:P18I10 VLPs. (A) Schematic process flowchart of L1:P18I10 VLP purification. The BEVS/IC expression system and ultracentrifugation (traditional method-1) were served as standard control methods in comparison with 293F expression system and chromatography (new method-2). (B) Characterization of BEVS/IC-derived L1:P18I10 VLPs purified by using SC and CsCl ultracentrifugation. L1:P18I10 VLP samples collected from different layers of SC and fractionated from CsCl gradients were analyzed by Coomassie-stained SDS-PAGE (left panel) and Western blot probed with HPV16 L1 mAb (right panel). The arrow indicates the molecular weight ~56 kDa of L1:P18I10 protein. Lane 1: protein molecular weight marker; Lane 2: clarified cell lysate (CCL); Lane 3: 0–20% interface of SC; Lane 4: 20–70% interface of SC; Lane 5: 70% tube bottom of SC; Lane 6: fraction-1 of CsCl; Lane 7: fraction-2 of CsCl; Lane 8: fraction-3 of CsCl. (C) Characterization of 293F-derived L1:P18I10 VLPs purified by using chromatography. L1:P18I10 VLPs produced in 293F cells underwent CEC, SEC and H-AC chromatography. Flow-though (FT) collected from different chromatographic purification steps were analyzed by Coomassie-stained SDS-PAGE gel (left panel) and Western blot probed with HPV16 L1 mAb (right panel). The arrow indicates the molecular weight ~56 kDa of L1:P18I10 protein. Lane 1: protein molecular weight marker; Lane 2: CCL; Lane 3: FT from CEC sample loading; Lane 4: CEC eluate; Lane 5: SEC FT; Lane 6: FT from H-AC sample loading; Lane 7: H-AC eluate; Lane 8: 10-fold diafiltration.