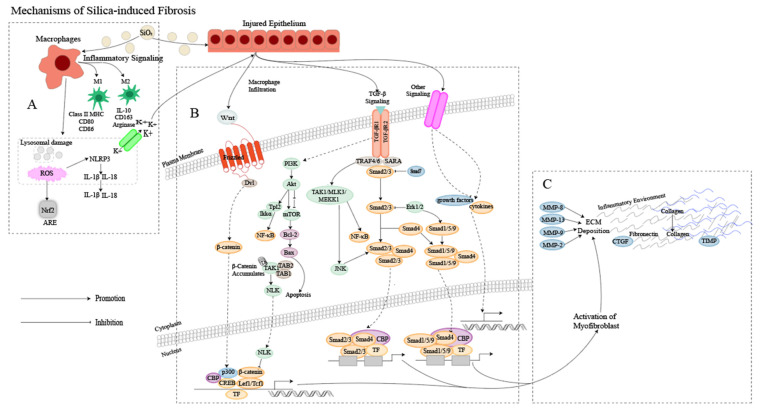
Figure 1.
Mechanism of silica-induced fibrosis. (A) Alveolar macrophages (AMs) engulf silica dust, causing them to turn into dust cells. Subsequently, AMs may synergize with alveolar epithelial cells (AECs) to release a large amount of ROS to participate in oxidative stress reactions, activate NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory bodies through lysosomal damage and potassium outflux, and activate the release of inflammatory mediator interleukin (IL) -1β, IL-18 and other cytokines inducing epithelial–mesenchymal transition (EMT). Meanwhile, AMs can polarize into M1 and M2 types, playing a role in promoting inflammation, fibrosis, and antigen presentation, increasing the proliferation of lung fibroblasts and collagen synthesis and secretion, and promoting the formation of fibrosis through apoptosis and autophagy. (B) Ongoing damage and damage to lung cells by silica lead to pathological overdeposition of extracellular matrix (ECM) proteins accompanied by upregulation of myofibroblast activity, resulting in a chronic inflammatory environment of macrophage and immune cell infiltration. In this cellular environment, cytokines and growth factors are released in large quantities, activating many signaling cascades, including members of the transforming growth factor-beta (TGF-β) family and Wingless/Int (Wnt) 1, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway and other pathways. (C) Fibroblasts then aggregate in the area of injury, and the combination of ECM degradation by MMPs and excessive collagen deposition leads to granuloma formation and lung tissue remodeling.