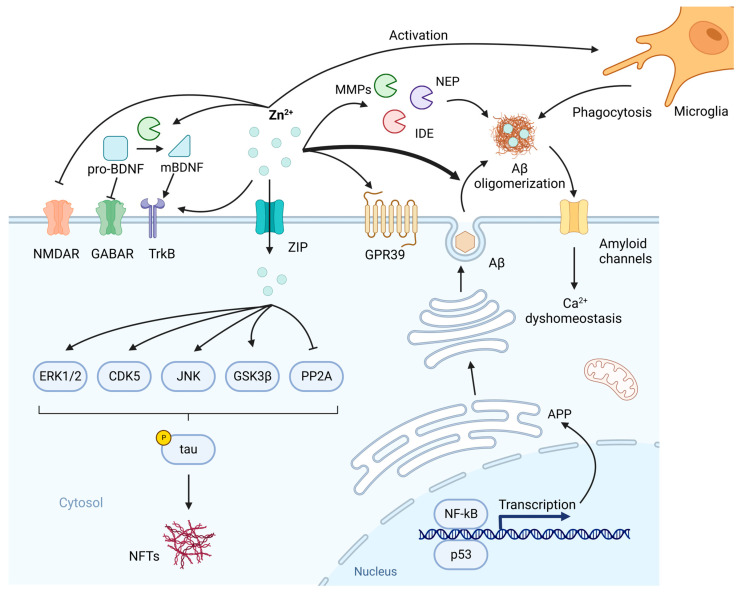
Figure 2.
The involvement of Zn2+ in the pathogenesis of Alzheimer’s disease (AD). Zn2+-containing transcription factors NF-κB and p53 participate in the synthesis of amyloid precursor protein (APP) and subsequent β-amyloid (Aβ). After production, Aβ is secreted upon neuronal activity and aggregated by Zn2+. In normal conditions, Zn2+ inhibits N-methyl-d-aspartate receptor (NMDAR) to regulate synaptic neurotransmission. Also, Zn2+ is involved in the normal memory events such as long-term potentiation (LTP) by TrkB and GPR39. brain-derived neurotrophic factor (BDNF)-TrkB axis needs to be modulated by Zn2+ because Zn2+-dependent MMPs are indispensable to the transformation of pro-BDNF to mBDNF, while pro-BDNF inhibits GABAergic transmission. In addition, the clearance of Aβ is affected by Zn2+. Zn2+ metalloproteinases including matrix metalloproteinases (MMPs), neprilysin (NEP) and insulin-degrading enzyme (IDE) are involved in the degradation of Aβ, while Zn2+-loaded Aβ oligomers have increased resistance to proteolysis. Microglia can be activated by Zn2+ to enhance the phagocytosis of Aβ. Due to the sequestration of Zn2+ by Aβ, these normal neurobiological activities in which Zn2+ plays a crucial role are disrupted. Aβ can incorporate into membranes to form amyloid channels, as a result, an inward flow of Ca2+ is initiated, leading to Ca2+ dyshomeostasis. On the other hand, intracellular Zn2+ can lead to phosphorylation of tau by activating extracellular signal-regulated protein kinase 1/2 (ERK1/2), cyclin-dependent kinase 5 (CDK5), c-Jun N-terminal kinase (JNK), glycogen synthase kinase 3β (GSK3β) and inhibiting protein phosphatase 2A (PP2A). Created with BioRender.com. Adapted from “Drosophila Toll Pathway”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates (accessed on 9 February 2023).