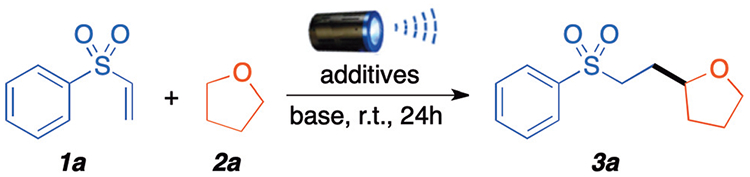
Table 1.
Optimization of the reaction and its conditionsa
| ||||
|---|---|---|---|---|
| Entrya | Additives (mol%) | Base (equiv) | Solvent | Yieldb (%) |
| 1 | Eosin Y (1%) | K2CO3 (2.0) | CH2Cl2 | 76 |
| 2 | — | K2CO3 (2.0) | CH2Cl2 | 40 |
| 3 | Eosin Y (1%) | — | CH2Cl2 | 50 |
| 4 | — | — | CH2Cl2 | 72 |
| 5 | — | — | CH2Cl2 | Tracec |
| 6 | — | — | CH2Cl2 | 40d |
| 7 | — | — | PhCH3 | Trace |
| 8 | — | — | MeCN | Trace |
| 9 | — | — | Acetone | Trace |
| 10 | — | — | Neat | 95(92)e |
| 11 | — | — | Neat | n.d.f |
| 12 | — | — | Neat | 80%g |
| 13 | — | — | Neat | 84%h |
| 14 | — | — | Neat | 86%i |
| 15 | — | — | Neat | 35%j |
Reaction conditions: 1a (0.2 mmol), THF 10 equivalent, solvent 1 mL, base 2.0 equivalent, room temperature, under argon atmosphere were irradiated with 40 W LED lamp (440 nm) for 24 h.
Yields are based on 1a, determined by 1H-NMR using dibromomethane as the internal standard.
Dark.
Open to air.
Isolated yields, 1 mL THF.
Reaction performed under 100% oxygen atmosphere.
Open to air conditions.
Under nitrogen atmosphere containing 1% oxygen.
Positive pressure of argon.
Freshly distilled THF, 24 hours, 440 nm blue LED.
