Abstract
The COVID-19 pandemic has highlighted the detrimental effect of secondary pathogens in patients with a primary viral insult. In addition to superinfections with bacterial pathogens, invasive fungal infections were increasingly reported. The diagnosis of pulmonary fungal infections has always been challenging; however, it became even more problematic in the setting of COVID-19, particularly regarding the interpretation of radiological findings and mycology test results in patients with these infections. Moreover, prolonged hospitalization in ICU, coupled with underlying host factors. such as preexisting immunosuppression, use of immunomodulatory agents, and pulmonary compromise, caused additional vulnerability to fungal infections in this patient population. In addition, the heavy workload, redeployment of untrained staff, and inconsistent supply of gloves, gowns, and masks during the COVID-19 outbreak made it harder for healthcare workers to strictly adhere to preventive measures for infection control. Taken together, these factors favored patient-to-patient spread of fungal infections, such as those caused by Candida auris, or environment-to-patient transmission, including nosocomial aspergillosis. As fungal infections were associated with increased morbidity and mortality, empirical treatment was overly used and abused in COVID-19-infected patients, potentially contributing to increased resistance in fungal pathogens. The aim of this paper was to focus on essential elements of antifungal stewardship in COVID-19 for three fungal infections, COVID-19-associated candidemia (CAC), -pulmonary aspergillosis (CAPA), and -mucormycosis (CAM).
Keywords: Antifungal stewardship, COVID-19-associated candidemia (CAC), COVID-19-associated pulmonary aspergillosis (CAPA), COVID-19-associated mucormycosis (CAM)
1. Introduction
The coronavirus disease-2019 (COVID-19) pandemic has gained the attention of healthcare workers (HCWs) around the world. Within this, the pandemic of opportunistic and multidrug-resistant organisms (MDROs) associated with COVID-19 must not be underestimated. Co-infections in COVID-19 patients have been reported [1,2], including infections with Staphylococcus aureus and Klebsiella pneumoniae, and many invasive fungal infections (IFIs), resulting in poorer outcomes [3,4]. Globally, the most commonly reported IFIs are candidemia and aspergillosis, and the prevalence of mucormycosis shows regional variation [5]. In a retrospective, multicenter, observational, cohort study from France, the mortality rate for critically ill COVID-19 patients with IFIs was 50.6%, compared with 22.6% for COVID-19 patients with no evidence of IFI [6]. Marked variation in the diagnostic capabilities of mycology laboratories in middle- and high-income countries further complicates reliable data capture [7,8]. The absence of a systematic approach to fungal diagnostics has led to over- or under-reporting of the problem [9].
It is difficult to diagnose IFIs in COVID-19 patients in the absence of pathognomonic clinical and radiographic findings on the background of lung injury secondary to the virus, coupled with limited sensitivity and specificity of diagnostic mycological tests. Distinguishing Aspergillus colonization from invasive disease is challenging. Contamination and clinically irrelevant colonization of the upper respiratory tract with fungi (mainly Aspergillus) was found in 17.2% of the COVID-19 patients admitted to five independent intensive care units (ICUs) from a single center [10]. Diagnostic uncertainty often leads to over-prescribing of antifungal agents, putting patients at risk for drug toxicity and drug interactions, and burdening institutions with high cost [11], [12], [13], [14]. Overuse of antimicrobial agents has been correlated with emerging resistance, and antifungal agents are no exception. This has been one of the most alarming examples of collateral damage resulting from the COVID-19 pandemic [15,16]. Antifungal-resistant IFIs may be devastating for hospitalized COVID-19 patients [17], with infections including echinocandin-resistant Candida glabrata [18,19], MDR Candida auris [17] and triazole-resistant Aspergillus fumigatus [20] bringing antifungal stewardship (AFS) into sharp focus.
Strategies for implementation of AFS are yet to be fully integrated into hospital protocols. The Mycoses Study Group Education and Research Consortium (MSGERC) recently addressed the application of the core elements of antimicrobial stewardship (AMS) to AFS, providing specific recommendations for developing interventions to measure and improve the appropriate use of antifungal agents [21].
This review focuses on AFS relevant to COVID-19. Although AFS practices that are applicable to the COVID-19 pandemic could be applicable to influenza pandemics, there are major differences in the presentation and outcome of some mold infections, such as aspergillosis, following the two viral infections. In addition, mucormycosis has not been widely reported following influenza. This paper presents the predominant fungal pathogens affecting COVID-19 patients, and discusses the role of screening, the timely diagnosis of infections, and the principles of optimal antifungal use along with tailored treatment strategies and patient monitoring. Also discussed is the prevention of IFIs as a core strategy of AFS. As the trauma of the COVID-19 pandemic gradually fades from public memory, and the political discourse shifts to other areas of healthcare, it is important that the wisdom acquired in relation to IFIs is used to improve diagnostic and therapeutic interventions, and infection control protocols.
2. Antifungal Stewardship diagnostics
Various fungal infections may occur in COVID-19 patients and an active diagnostic strategy should be pursued [22]. Blood culture remains the cornerstone for the diagnosis of yeast bloodstream infection (BSI), but the yield of blood culture remains suboptimal; (1,3)-β-D-glucan (BDG) detection may enhance the diagnosis of BSI [23], [24], [25]. COVID-19-associated pulmonary aspergillosis (CAPA) is often diagnosed based on biomarker test and culture results from respiratory tract samples, as radiological findings are commonly non-specific. Also, serum galactomannan (GM) is positive in a minority (typically less than 15%) of patients [23,26,27]. The diagnosis of CAPA has proven difficult due to the frequency of positive cultures from the upper respiratory tract (e.g., sputum and tracheal aspirates), which may reflect colonization rather than invasive disease. Lung biopsy provides definitive diagnosis but is not normally undertaken due to associated risks. Bronchoscopy and lavage are widely used as diagnostic interventions, with the associated risk of aerosol generation. The European Confederation of Medical Mycology and the International Society for Human and Animal Mycology support the use of polymerase chain reaction (PCR) assay to estimate the contagiousness of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The PCR assay results may be used to guide the appropriateness of bronchoscopy without putting HCWs and other patients at risk of viral transmission. In centers with limited facilities for bronchoscopy, tracheal aspirates can reveal a mold, but it may not be indicative of pulmonary tissue infection. The usefulness of sputum culture is even more limited. Thus, sampling of lower respiratory tract by bronchoscopy is generally recommended to diagnose CAPA. A positive bronchoalveolar lavage (BAL) culture and/or positive BAL-biomarkers, such as Aspergillus GM or BDG, do not confirm the presence of invasive disease [28], but the diagnosis of CAPA must be considered.
BDG is a cell wall component present in many fungi. Basidiomycetes, the Mucorales, and Blastomyces species lack or have low quantities of BDG. BDG has a high negative predictive value (NPV) for diagnosis of aspergillosis and candidiasis and its absence excludes an IFI in a setting with low clinical suspicion. In practice, BDG is mostly used for the diagnosis of invasive candidiasis (IC), as there are specific tests, such as GM, available for diagnosing invasive aspergillosis (IA). Additional mycological tests are required to identify pathogens with certainty.
The use of BDG to detect IFI may be an attractive concept, but the proportion of CAPA patients with positive serum biomarkers is relatively low [23,29]. Therefore, fever and clinical or respiratory deterioration are the triggers for fungal diagnostic work-up [22,28]. There is overlap in the timing of various secondary IFIs, although the median time to CAPA and COVID-19-associated mucormycosis (CAM) and that of COVID-19-associated candidemia (CAC) is 7 and 15 days (interquartile range, 8–21) post ICU admission, respectively [5,30,31].
Computed tomography scan of the chest seldom shows specific lesions [28]. However, when multiple pulmonary nodules or lung cavitation are present, a diagnostic work-up for fungal pneumonia is recommended, as these appearances are rarely associated with SARS-CoV-2 infection [28]. In a case series of 12 patients with pulmonary CAM, a reversed halo sign was observed in 1 patient, consolidation in 10 patients, including cavitation in 4, and pulmonary nodules in 1 [32]. Given the lack of specific radiological images, the diagnosis of secondary pulmonary mold infections relies on bronchoscopy and BAL [22,28].
Invasive Aspergillus tracheobronchitis may be present in up to 47% of COVID-19 ICU patients with CAPA; therefore, bronchoscopic inspection of trachea and bronchi is required [27]. In patients with endotracheal plaques, a mucosal biopsy is recommended to demonstrate the presence of tissue invasion by Aspergillus [22]. If invasive procedures are precluded due to thrombocytopenia, the use of a brush can be considered. Positive BAL culture, GM, or Aspergillus PCR in the clinically deteriorating, critically ill COVID-19 patient are considered sufficient evidence to initiate antifungal therapy. Presence of serum GM is a marker of angioinvasion, indicating advanced disease [33]. One study showed 86% mortality in serum GM-positive CAPA patients and 90% morality in serum BDG-positive patients, compared with 38% in those who remained biomarker negative [29]. In other studies, high BAL-GM, positive serum GM, and multiple positive markers in BAL (i.e., microscopy, BAL-GM and Aspergillus PCR), were associated with increased mortality [29,34,35].
The Aspergillus Lateral Flow Assay (LFA) has several advantages, including ease of use, single sample testing, rapid availability of results, and good concordance with GM. The LFA showed good diagnostic performance for CAPA diagnosis using respiratory samples at the 1.0 cutoff in a retrospective multicenter study. Sensitivity of LFA for CAPA was 52%, 80% and 81%, and specificity was 98%, 88% and 67%, for BAL fluid, nondirected BAL, and tracheal aspiration, respectively. There was an increased sensitivity of 72%, 90% and 100%, but a reduced specificity of 79%, 83% and 44%, respectively, at a 0.5 cutoff. The sensitivity of serum LFA is limited (20% and 9% at the 0.5 cutoff and 1.0 cutoff, respectively), and is probably linked to weak invasiveness during CAPA [36]. Processing of potentially infectious respiratory samples, including those for LFA testing, should take place in appropriate facilities (ideally in a category 2/3 safety cabinet) as per good laboratory practice, to protect staff and patients from infection, and the sample from contamination.
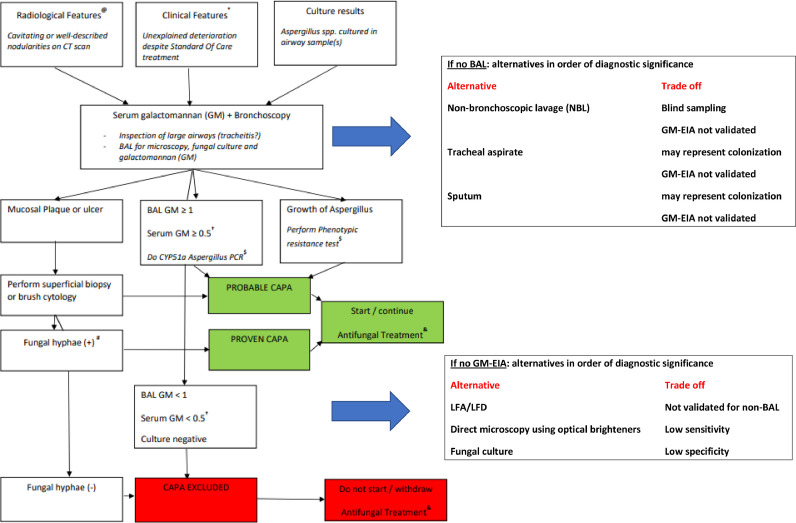
Fig. 1 provides an integrated approach to therapy, triangulating the clinical, radiological, and laboratory findings of CAPA.
Fig. 1.
An integrated approach to antifungal therapy in COVID-19
(@) This does not mean that a lung CT should be standard of care for all ICU patients with COVID-19. Instead, the flow diagram is meant to be used when a CT is done during routine patient care and shows cavitating or well-described nodular lung lesions.
(*) Standard of care. The SOC of COVID-19 is likely to change in the future but for now it includes thromboembolic prophylaxis, therapy with dexamethasone, and exclusion of pulmonary embolism with CT. Other causes of clinical respiratory deterioration may also need to have been excluded: pneumothorax, atelectasis, and progressive pulmonary fibrosis.
($) If there is growth of Aspergillus, phenotypic resistance testing can be used, e.g., with VIPcheck on site or at a mycology reference laboratory. In culture-negative but GM-positive BAL samples, the CYP51A Aspergillus PCR can be used to exclude the presence of the two most frequent resistance mutations that confer azole resistance (TR34/TR46 pattern).
(#) Formally, only when septate hyphae of 2.5–4.5 µm in diameter are seen AND the presence of Aspergillus DNA is documented, the infection is classified as proven CAPA. However, the presence of hyphae compatible with Aspergillus suffices to start antifungal therapy.
(†) Serum GM is generally negative, but increases the probability of CAPA if it is positive in combination with positive BAL GM.
(&) It is recommended to start antifungal therapy as early as possible. If BAL test results are available the same day, these can be awaited before antifungal therapy is started. If BAL test results are not immediately available, it is recommended to consider starting antifungal therapy pre-emptively while awaiting test results.
Mucorales are fragile and easily damaged during standard respiratory sample processing. Also, they cannot be detected using GM and BDG tests. Invasive procedures are considered critical to diagnose mucormycosis [37]; however, targeted lung biopsies are generally not conducted in COVID-19 patients due to the frequent absence of specific lung lesions on imaging. In a retrospective study from France, Mucorales PCR assay of serum was positive in 14 of 17 (82%) CAM patients, indicating that PCR may be of value in this setting [32]. Unlike with aspergillosis, respiratory tract recovery of Mucorales is uncommon in ICU patients [38]. Detection of Mucorales in a respiratory sample should prompt a full diagnostic work-up, even in patients with CAPA as dual infections have been reported [32,39].
3. Treatment strategies
3.1. Background and treatment of common IFIs
Azoles, echinocandins, and amphotericin B (AmB) are commonly used to treat IFI in COVID-19 patients [40]. Although patients with positive Aspergillus cultures or GM have survived without receiving antifungal therapy [23,26,28], ICU mortality associated with secondary fungal infections in severe COVID-19 is reported to be more than 50%, and this is an incentive to start antifungal therapy in patients with positive fungal cultures and/or biomarkers. As the diagnosis of IFI is challenging, empirical antifungal therapy based on suspicion of infection, without laboratory confirmation, is sometimes used, particularly in critically ill patients. The clinical scenarios would differ in the settings of CAC, aspergillosis and mucormycosis. The optimal management of secondary fungal infections in these settings depends on the risk profile of the COVID-19 ICU patients, the changing virulence of SARS-CoV-2, the use of antivirals and immunosuppressants, and the fungal resistance profile. Table 1 lists first-line and alternative recommendations to treat common IFIs.
Table 1.
| Fungal infections | First-line drugs | Alternative drugs |
|---|---|---|
| Candidiasis | Echinocandins (anidulafungin, caspofungin, micafungin) |
|
| Aspergillosis | Isavuconazole, voriconazole |
|
| Mucormycosis | Lipid formulations of amphotericin B |
|
If lipid formulations are not available.
3.1.1. Invasive Candidiasis
The most common causative species of fungal infections is Candida albicans, which is almost always susceptible to azoles and echinocandins. Resistance to echinocandins is mainly reported for C. glabrata in North America [43]. High rates of azole resistance in C. glabrata and intrinsic azole resistance in Candida krusei are well known [43], [44], [45]. C. auris has been reported in COVID-19 patients, including in centers that had never reported cases prior to the pandemic [46,47].
Echinocandins remain the drugs of choice for suspected IC. Fluconazole is an acceptable alternative initial therapy in regions with low resistance in patients who are not critically ill. AmB is an acceptable alternative in critically ill patients, in scenarios of known resistance to echinocandins, or in low- and middle-income countries where echinocandins are not readily available. It is also acceptable as a step-down therapy, taking into account the problems of toxicity and the need for monitoring. In some clinical syndromes (central nervous system [CNS] infection, candiduria, and endophthalmitis), azoles are preferred. AmB lipid (L-AmB) formulations are preferred over AmB deoxycholate as they enable higher doses to be administered without increasing nephrotoxicity [25].
Switching to azoles is driven by susceptibility pattern and clinical improvement [25]. In clinically stable patients, empirical therapy can be withheld and should be discontinued if alternative diagnoses are established.
3.1.2. Pulmonary Aspergillosis
Resistance of A. fumigatus to azoles has been reported, with marked variation in prevalence between countries [48]. CAPA appears to follow the local epidemiology of IA where data are available. The lack of data is mainly due to avoidance of bronchoscopy and other aerosol-generating procedures in the setting of COVID-19 [49], [50], [51]. In addition, testing for resistance is not routinely performed in many centers, so estimating the prevalence of azole resistance in CAPA is difficult.
Antifungal therapy is generally recommended in clinically deteriorating COVID-19 patients with evidence of Aspergillus in BAL [22]. The azoles are the most commonly used class, with voriconazole and isavuconazole being the azoles of choice, and posaconazole as an alternative [52]. Considering pharmacokinetic (PK) and pharmacodynamic (PD) issues, itraconazole should only be used in patients with less severe disease and when other azoles are not available [41].
AmB and L-AmB are alternatives for initial and salvage therapy when voriconazole or other azoles cannot be administered, or when the minimum inhibitory concentration (MIC) of voriconazole is >2 mg/L. Caspofungin or micafungin can be used when azoles and AmB are contraindicated [41,53], or as salvage therapy.
If simultaneous mucormycosis infection is suspected, L-AmB, isavuconazole or posaconazole are preferred. Several studies have investigated alternative management strategies, including the use of nebulized or systemic antifungal prophylaxis and diagnostic driven strategies, but firm conclusions cannot be drawn at this stage [41,53]. Only six studies to date have looked into antifungal prophylaxis for CAPA [54], [55], [56], [57], [58], [59]. Posaconazole, administered through a gastric tube or intravenously, and inhaled AmB have been investigated. Prophylaxis significantly reduced the incidence of CAPA in three of these investigations [54], [55], [56], but did not influence survival in the two studies [54,55] that reported survival data. There are currently insufficient data to recommend prophylaxis. Nonetheless, in hospitals where these infections are common (e.g., 15–30%), prophylaxis can be considered.
3.1.3. Mucormycosis
Mucoraceous molds are a broad group of fungi that are intrinsically resistant to voriconazole, the first-line treatment for IA, but are usually susceptible to posaconazole and isavuconazole. L-AmB is the treatment of choice. The therapeutic place for this drug is treatment of viral-associated mucormycosis, triazole-resistant aspergillosis, or if there is intolerance to triazoles or echinocandins.
For the management of non-pulmonary manifestations of CAM, treatment initiation with L-AmB and surgical debridement to clean margins is recommended. Isavuconazole or posaconazole can be used as step-down therapy [37].
3.2. Specific recommendations
3.2.1. Combination therapy
Empirical use of combination therapy is discouraged. Attention should focus on compliance and other factors, as mentioned above, if adequate drug levels are not attained. Reversal of immunosuppressive status, whenever possible, should also be considered when managing difficult-to-treat IFIs. However, dexamethasone and interleukin-6 inhibitors (tocilizumab or sarilumab) should not be discontinued in COVID-19 patients without expert advice. Combination therapy may sometimes be required to treat MDR Candida spp. and A. fumigatus, infections of the CNS or endocarditis, in salvage therapy for IA (combination of echinocandins with voriconazole) or when treating rare mold infections [60]. For aspergillosis, combining voriconazole with an echinocandin may be considered for patients who are not responding to monotherapy, in serious presentations in critically ill patients, or when azole resistance is suspected. Concomitant therapy with azoles and AmB remains controversial as azoles may decrease AmB-binding sites. For mucormycosis, combination therapy (isavuconazole or posaconazole, with AmB) is not supported by clinical data, although it has been used in severely immunosuppressed patients with variable results [60].
3.2.2. Therapeutic drug monitoring
Therapeutic drug monitoring (TDM) of antifungals is expensive, time consuming, and not fully supported by evidence. However, the costs of TDM should be weighed against the costs of diagnosis and treatment of IFI. Also, recent studies have shown that in critically ill patients, antifungal drug exposure is unpredictable due to altered PK and PD [61], [62], [63], [64]. TDM for azoles has been shown to have potential clinical benefit but is not recommended for polyenes and echinocandins [65]. Fluconazole has linear PK and high oral bioavailability, therefore TDM is not indicated except when treating CNS infections, pathogens with high MIC, or in patients on renal replacement therapy [66]. On the other hand, itraconazole has non-linear PK and variable bioavailability, hence TDM is recommended [66]. Voriconazole has numerous drug-drug interactions and attains variable levels, necessitating TDM [67]. Plasma levels of 1–5.5 mg/L are considered adequate for most patients [52] and the trough concentration to minimize drug-related toxicity is <4 mg/L [66]. Higher trough levels (2–6 mg/L) are recommended in patients with severe infections (multifocal or disseminated disease, CNS infections, infection with pathogens with elevated MICs, i.e., >2 mg/L) [52,66].
TDM is recommended for posaconazole as it has many drug-drug interactions, and food-related factors affect oral absorption of the liquid formulation. A target concentration >0.7 mg/L for prophylaxis and >1 mg/L for treatment is recommended [68]. There are limited data to define a target therapeutic range or support the need for routine TDM for isavuconazole; however, TDM for isavuconazole may be indicated in patients who remain unresponsive, have unexpected toxicity, or have difficult-to-treat conditions (e.g, CNS infections). A plasma trough concentration of 2–3 mg/L is considered adequate after day 5 of exposure [52]. There is limited support for first-line use of isavuconazole [69].
Although there is no indication for TDM for AmB or L-AmB, patients should be carefully monitored (2–3 times weekly) for cell count, electrolytes, serum magnesium, and serum creatinine. Progressive steps to target TDM are displayed in Table 2 .
Table 2.
Progressive steps to perform when therapeutic drug monitoring is not available
|
3.2.3. Duration of treatment
Table 3 summarizes the duration of therapy for IFI in the setting of COVID-19.
Table 3.
Suggested treatment duration for common fungal infections.
| Condition | Treatment duration |
|---|---|
| Uncomplicated invasive candidiasis | Two weeks after the first negative blood culture [70] |
| Complicated invasive candidiasis (e.g., sanctuary sites, endocarditis) | Three to 12 months, depending on clinical improvement and assessment of the local site (e.g., fundus examination, cerebrospinal fluid parameters in case of meningitis) and radiological images (magnetic resonance imaging or computed tomography etc.), if available [24,25,71] |
| Pulmonary aspergillosis | Six to 12 weeks, provided there is clinical and radiological improvement [72] |
| Disseminated aspergillosis | Up to 12 months, depending on the localization and possibilities of surgical removal [41,69] |
| Mucormycosis | Complete surgical debridement and at least 2 weeks of amphotericin B lipid formulation, followed by posaconazole or isavuconazole for 6–12 weeks [42,73] |
4. Infection control
With multiple reports of fungal outbreaks during the COVID-19 pandemic, it is essential not to underestimate the role of infection control in healthcare settings [74]. Although the pathogenesis of these fungal infections in COVID-19 patients is not fully elucidated, a rigorous understanding of their spread helps to guide some important preventive measures. Standard precautions, such as those used for MDROs are recommended [75,76]. However, this is a minimum requirement, and HCWs should use a medical mask, long-sleeved gowns, and gloves to avoid auto-inoculation.
Most patients with severe COVID-19 require ICU admission and immunosuppressive therapy, central venous catheter, parenteral nutrition, and mechanical ventilation. These factors, along with advanced age and high Sequential Organ Failure Assessment score, have been linked to a higher risk of candidemia [77], [78], [79], [80], which is challenging for the management of these patients, particularly in the setting of a pandemic [81]. Nevertheless, adherence to evidence-based central line-associated BSI prevention bundles can reduce candidemia, alongside the early removal of the catheter, whenever possible [82,83].
C. auris is a growing healthcare threat [17,84]. Hospitals should have guidelines for the rapid detection and prevention of C. auris, including screening and isolation on admission of all patients at risk [85], such as patients transferred from institutions with ongoing outbreaks. Fine tuning this strategy to only ICU patients may be needed, depending on local resources and epidemiology [86]. Axilla and groin are favored screening sites; however, institutions may choose to rely on their standard screening protocols, including sites such as nose, mouth, rectum, open wounds, catheter sites, or urine [87]. To minimize time spent in isolation, initial screening may be done by PCR, if possible [88], followed by culture, for epidemiological investigation. C. auris may remain deep within the skin tissue compartment for a considerable period of time, even when the skin surface swabs are repeatedly negative [89]. Patients colonized or infected with C. auris should be isolated until discharge and flagged for at least one year after the first negative screening culture. Alcohol-based hand rubs have been shown to be effective against C. auris [76], and HCWs should be reminded of the importance of adequate hand hygiene, including appropriate use of medical gloves. Despite potent in vitro activity [90], chlorhexidine (CHG) bathing showed little effect in an ex vivo skin model [91], possibly explaining the clinical failure of earlier attempts to decolonize patients [92]. In contrast, a recent in vivo study demonstrated the protective effect of CHG against C. auris colonization of both skin surface and skin tissue in murine models. Whether these results are applicable to the use of CHG bathing to decolonize patients requires further evidence from clinical trials [89]. Rigorous environmental cleaning is essential. Chlorine-based products are favored [93], but other products such as iodinated povidone, hydrogen peroxide vapor, and Ultraviolet-C have been found to be effective at concentrations used in clinical practice [86,94].
Air room pressure is an essential part of controlling the spread of infectious diseases within hospitals and this became a hot topic during the COVID-19 pandemic. For patients on oxygen supplementation that generates aerosols, such as high-flow oxygen, continuous positive airway pressure or bilevel positive airway pressure, the Centers for Disease Control and Prevention recommends the use of negative pressure rooms (NPR) with an anteroom to ensure safety of other patients, visitors, and HCWs [95,96]. However, the risk of acquiring superimposed infections, such as IA has not been specifically addressed. Positive pressure rooms provide a ‘protective environment’ (PE) by pressing particles out of the room, without them circulating back in [97]. PE-rooms may be preferred in wards with large numbers of immunocompromised patients to protect them from Aspergillus spp. [98]. In a study conducted in 15 ICU rooms in France, NPR was shown to increase the risk of airborne mold infections. Air-cultures from NPR were positive for Aspergillus spp., and probable or proven pulmonary aspergillosis developed in six patients. After a switch to a slightly positive pressure (1.2 ± 1.5 Pa), the number of Aspergillus colonies markedly diminished and ultimately became undetectable, thereby theoretically reducing the possibility of developing IA [99]. The incidence of CAPA is variable across centers, and more research is needed to determine how common these infections are among COVID-19 patients. This information will be useful for providing tailored infection control guidelines for this cohort of patients. In the meantime, exposure to airborne Aspergillus can be minimized by implementing recommendations on environmental controls, using well-sealed rooms, and controlling air quality. Air-filtration using mobile high efficiency particulate air (HEPA) filters could reduce the load of aerosols in rooms with COVID-19-infected patients. Whether these systems are effective in preventing colonization with Aspergillus needs to be examined [100,101].
The burden of Mucorales spores in the hospital and outdoor environment was evaluated in several Indian centers during the pandemic [102], [103], [104]. A multicenter research study showed a significant Mucorales spore count in the hospital air and air-conditioning ducts, with a significantly higher burden in the air of rooms with personalized air cooling (AC) compared with that in rooms with central AC with connected microfilters. Mucorales spore count was also used at one center to measure the effect of cleaning the AC filters of five window ACs, and showed that washing with soap and water reduced spore counts from a pre-cleaning value of 24.8 ± 10.5 to 1.7 ± 1.2 cfu/m3 [104], with similar findings in a hematological ICU from a different center [102]. Moreover, rooms with HEPA-filtered air had significantly less contamination (2.1%) than rooms without HEPA filters (20.5%), but spores were not isolated from hospital equipment and surfaces [104]. A large proportion of patients acquired CAM during convalescence at home, prompting an environmental assessment for Mucorales in their domestic environment [103]. Based on these findings of Ghosh et al., it would seem prudent to advise patients with a high risk of acquiring mucormycosis, such as those in the COVID-19 convalescence phase, to wear an appropriate mask even at home during the risk period. Further studies are warranted to ascertain the duration for masking. Regular aeration of quarantine rooms and exposure to sunlight may also potentially help reduce spore dispersal [103]. Prompt glycemic control, management of ketoacidosis and hypoxia, attention to leucopenia, and judicious use of corticosteroids, immunomodulators, and antimicrobials are all important for decreasing mucormycosis occurrence [105]. Of note, in a case control study from India, prolonged use of cloth and surgical masks (more than 4 and 6 h, respectively) and repeated nasopharyngeal swab testing during the COVID-19 illness were both independent variables of developing CAM. N95 masks and zinc therapy were found to be protective [106].
5. Antifungal stewardship: Quality improvement
The aim of AMS is to reduce inappropriate antimicrobial prescribing, minimize toxicity, limit selection pressure, and reduce costs [107]. More recently, AFS has gained attention following the emergence of triazole-resistant A. fumigatus and outbreaks of C. auris.
In a survey of AFS initiatives across hospitals in England, only 5 (11%) responders had a dedicated AFS program, and 20 (43%) had AFS included as part of their AMS program. Perceived benefits of AFS included improvements in safety, outcome and costs, reduced side-effects, and collection of surveillance data [108]. However, the impact of these programs on patient outcome remains elusive. In a systematic review of 13 studies on the impact of AFS interventions in the United States, improvement in clinical outcomes was not detected; however, the results indicated that AFS interventions improved appropriate antifungal choice and time to therapy, and decreased antifungal consumption [109].
Although the principles of AMS apply to the management of fungal infections, there are additional factors to consider. For example, patient-to-patient transmission is rare and fungal infections are more often acquired from the environment, the patient's own flora, or devices such as catheters. In addition, toxicity and drug–drug interactions are more common, biomarkers have variable sensitivity and specificity, and diagnostic tools are less available and are difficult to interpret [108].
Performance assessment followed by tangible measures enable a formal review of intervention, allow benchmarking, and serve as a quality improvement (QI) tool. The classification of performance assessment into process, outcome, and structural measures improves the understanding of quality assurance. Process measures refer to the main diagnostic and therapeutic aspects of QI, such as streamlining the use of antifungal agents and appropriate utilization of diagnostic tools, whereas drug expenditure is an outcome measure. Structural measures are broad-based and conform to the overall strategy, e.g., adoption of antifungal policies by hospitals [110].
Eighty-two experts from 17 countries participated in a series of web-based questionnaires to assess the significance and feasibility of metrics in AFS using Delphi technique. The basic AFS indicators provide a foundation for developing QI programs targeted at improving IFI prevention, management, and patient-centered outcomes by systematically assessing the quantity and quality of antifungal medication within hospitals [111]. The effectiveness of AFS programs must be tracked using simple-to-measure variables, such as the prevalence and mortality of nosocomial IFI, the rate of fluconazole resistance, and the use and cost of antifungal medications. The Collaborative Group on Mycoses conducted point prevalence audits on 100 consecutive patients receiving systemic antifungals, with each day of therapy being assessed in accordance with a pre-established score that enabled individualized evaluation of the key elements of drug usage (need for antifungal therapy, selection of the drug, dose and administration route, adjustment to microbiology results and duration of therapy). The group demonstrated that the AFS program resulted in a definite improvement through educational initiatives, local guidelines, adoption of novel diagnostic techniques, and professional clinical guidance and audits. To help clinicians feel secure when stopping empirical antifungal treatment and excessively prolonged therapy, the group suggested using a combination of biomarkers - BDG and C. albicans germ tube assay - performed on days 0, 3, and 5 of empirical antifungal therapy, which showed a very high NPV (97% for the general population and 100% in ICU patients) [112]. A systematic review showed that a reduction in antifungal consumption was the single most effective measure in relation to impact assessment of AFS [113]. A clinical pharmacist-led candidemia treatment bundle implemented in a tertiary care facility in India significantly increased the appropriateness of antifungal prescriptions from 30% to 65% in the post-implementation period, with a reduction in in-hospital mortality rate from 40% to 36% (P=0.26; not significant), demonstrating successful implementation of an AFS program in a low-middle income country setting [114]. In a study from Greece that assessed the impact of the implementation of a non-compulsory AFS program with educational intervention to increase the awareness on proper use of antifungals, statistical analysis revealed a large, immediate decline in improper prescriptions and total consumption following intervention, with a downward trend thereafter. When pre- and post-interventional periods were compared, there was a considerable reduction in acquisition costs but no difference in in-hospital mortality or mean in-hospital length-of-stay [115]. Reviewing prescriptions at least three times a week, particularly for more expensive agents, and pre-authorization for these agents are effective in rationalizing and reducing their use. A fully functional AFS team is crucial for the successful implementation of AFS programs but many hospitals do not have such a team. The incorporation of PK and PD modeling in AFS needs further research [116].
One of the drawbacks of AFS is the limited epidemiological data in relation to antifungal resistance. In parallel, there is increasing use of antifungal agents in agriculture and farm animals, which predisposes fungi to become resistant even before they infect humans [117]. Together, the increase in fungal infections and rising level of resistance create a threat that can no longer be ignored. Indeed, mutations that define azole resistance are found in clinical and environmental Aspergillus [118] and in many Candida spp. [85]. Considering these facts, ideally all invasive yeasts and molds should be identified to the species level and subjected to susceptibility testing. However, the Infectious Diseases Society of America does not recommend routine MIC testing for Aspergillus spp. (only in case of treatment failure) and little is known of the usefulness of MIC testing of Mucorales [41]. Where local susceptibility testing is not possible, isolates should be referred to a regional reference laboratory. The value of routine screening of patients for C. auris is uncertain. When there is low background prevalence of C. auris, universal screening for this fungus is not recommended but can be targeted to high-risk groups [119,120].
Pre-authorization, post-authorization review, audit, promoting local guidelines, and early intravenous-to-oral switch form the basis of stewardship [121]. Whitney and colleagues found that as many as 25% of patients who received antifungal agents as part of ‘targeted’ therapy did not have proven, probable or possible IFI based on European Organisation for Research and Treatment of Cancer/MSGERC criteria, and a significant number of patients who received empirical antifungal therapy did not have fungal infection on retrospective analysis [122]. AFS needs to focus on this overuse. The COVID-19 pandemic led to an unprecedented interest in fungal infections [123]. Yet, research in mycotic diseases is underfunded in many countries, such as the UK, which has an allocation of only 2% of the budget for infectious diseases [124]. A One Health approach, using integrated data platforms, such as EpiCollect and EpiCollect plus, is the way forward [125].
QI is also applicable to environmental sampling. If an unexplained cluster of cases of nosocomial IA is detected, an environmental audit should be carried out to detect any linked cases within a stipulated time frame. Genotyping of clinical and environmental isolates may point to a common source, with some evidence showing a correlation between aerial fungal counts and occurrence of fungal infection [126].
6. Conclusions
The COVID-19 pandemic has increased awareness of clinical manifestations associated with the broad spectrum of fungal pathogens. Fungal infections are over-suspected and under-diagnosed. Reports on the presence of fungal disease associated with COVID-19 have varied widely because of the difficulties in differentiating colonization from infection. Under both circumstances, and independent of the causative pathogen, the use of antifungal drugs has increased. The uncertainty of diagnosis has made clinicians err on the safe side and initiate antifungal therapy at an early stage. This has led to spiraling and indiscriminate use of antifungal agents. To curb overprescribing, adhering to the principles of AFS is vital. A multidisciplinary team that benefits from the expertise of the intensivist, pulmonologist, infection specialist, and clinical pharmacist is desirable for optimal delivery of AFS. AFS teams should incorporate experts in fungal infections and should regularly evaluate performance on relevant structural, process and outcome measures, and implement evidence-based strategies in a continuous quality of care improvement cycle. In this process, it is essential to not disregard the role of infection control to alleviate the burden of these infections. All these elements need to be integrated in a bundle approach (Table 4 ). It is imperative to remain alert and continue to fight against this developing global pandemic of drug-resistant fungi.
Table 4.
Minimum bundles for the management of prevalent fungal infections
Candidiasis
|
Declarations
Funding: No funding was received for the conception of this manuscript
Competing Interests: All authors declare potential conflict of interest regarding the manuscript as noted below:
Souha S. Kanj Pfizer, MSD, Menarini, Basilia, Gilead personal funds
Sara F. Haddad NO conflict of interest
Jacques F. Meis NO conflict of interest
Paul E. Verweij F2G, Gilead, Pfizer and Mundipharma funds all paid to institution
Andreas Voss NO conflict of interest
Riina Rautemaa-Richardson Pfizer, Mundipharma, Gilead personal funds
Gabriel Levy-Hara Advisory board of Biomerieux / Roche Diagnostics Latin America
Anuradha Chowdhary NO conflict of interest
Abdul Ghafur NO conflict of interest
Roger Brüggemann NO conflict of interest
Abhijit M. Bal Pfizer, Astellas, Gilead, Menarini, Tillots
Jeroen Schouten NO conflict of interest
Ethical Approval: Not required.
Sequence Information: Not applicable.
Editor: Professor Philippe Colson
Footnotes
A consensus statement on behalf of the International Society of Antimicrobial Chemotherapy, Alliance for the Prudent Use of Antibiotics, European Society of Clinical Microbiology and Infectious Diseases Study Group for Antimicrobial Stewardship, and European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group.
References
- 1.Clancy CJ, Nguyen MH. Coronavirus Disease 2019, Superinfections, and antimicrobial development: what can we expect? Clin Infect Dis. 2020;71:2736–2743. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llitjos J, Bredin S, Lascarrou J, Soumagne T, Cojocaru M, Leclerc M, et al. Increased susceptibility to intensive care unit-acquired pneumonia in severe COVID-19 patients: a multicentre retrospective cohort study. Ann Intensive Care. 2021;11:20. doi: 10.1186/s13613-021-00812-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sreenath K, Batra P, Vinayaraj EV, Bhatia R, SaiKiran K, Singh V, et al. Coinfections with other respiratory pathogens among patients with COVID-19. Microbiol Spectr. 2021;9 doi: 10.1128/spectrum.00163-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux J, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3:e543–e552. doi: 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretagne S, Sitbon K, Botterel F, Delliere S, Letscher-Bru V, Chouaki T, et al. COVID-19-associated pulmonary aspergillosis, fungemia, and pneumocystosis in the intensive care unit: a retrospective multicenter observational cohort during the first French Pandemic Wave. Microbiol Spectr. 2021;9 doi: 10.1128/Spectrum.01138-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmanton-Garcia J, Hoenigl M, Gangneux J, Segal E, Alastruey-Izquierdo A, Arikan Akdagli S, et al. The current state of laboratory mycology and access to antifungal treatment in Europe: a European Confederation of Medical Mycology survey. Lancet Microbe. 2023;4:e47–e56. doi: 10.1016/S2666-5247(22)00261-0. [DOI] [PubMed] [Google Scholar]
- 8.Salmanton-Garcia J, Au W, Hoenigl M, Chai LYA, Badali H, Basher A, et al. The current state of laboratory mycology in Asia/Pacific: A survey from the European Confederation of Medical Mycology (ECMM) and International Society for Human and Animal Mycology (ISHAM) Int J Antimicrob Agents. 2023;61 doi: 10.1016/j.ijantimicag.2023.106718. [DOI] [PubMed] [Google Scholar]
- 9.Prattes J, Koehler P, Hoenigl M, ECMM-CAPA Study Group COVID-19 associated pulmonary aspergillosis: regional variation in incidence and diagnostic challenges. Intensive Care Med. 2021;47:1339–1340. doi: 10.1007/s00134-021-06510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin J, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med. 2021;203:307–317. doi: 10.1164/rccm.202009-3400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halpern AB, Culakova E, Walter RB, Lyman GH. Association of risk factors, mortality, and care costs of adults with acute myeloid leukemia with admission to the intensive care unit. JAMA Oncol. 2017;3:374–381. doi: 10.1001/jamaoncol.2016.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz P, Valerio M, Vena A, Bouza E. Antifungal stewardship in daily practice and health economic implications. Mycoses. 2015;58(Suppl 2):14–25. doi: 10.1111/myc.12329. [DOI] [PubMed] [Google Scholar]
- 13.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valerio M, Rodriguez-Gonzalez CG, Munoz P, Caliz B, Sanjurjo M, Bouza E, et al. Evaluation of antifungal use in a tertiary care institution: antifungal stewardship urgently needed. J Antimicrob Chemother. 2014;69:1993–1999. doi: 10.1093/jac/dku053. [DOI] [PubMed] [Google Scholar]
- 15.Arastehfar A, Lass-Florl C, Garcia-Rubio R, Daneshnia F, Ilkit M, Boekhout T, et al. The quiet and underappreciated rise of drug-resistant invasive fungal pathogens. J Fungi (Basel) 2020;6:138. doi: 10.3390/jof6030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai C, Chen S, Ko W, Hsueh P. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021;57 doi: 10.1016/j.ijantimicag.2021.106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg Infect Dis. 2020;26:2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brikman S, Dori G, Kasher C, Yanovskay A, Strauss M, Colodner R, et al. Candida bloodstream infection, a dire complication in hospitalized COVID-19 patients: three cases from a single center in Northern Israel. Isr Med Assoc J. 2021;23:615–617. [PubMed] [Google Scholar]
- 19.Posteraro B, Torelli R, Vella A, Leone PM, De Angelis G, De Carolis E, et al. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: a fatal case report. J Fungi (Basel) 2020;6:163. doi: 10.3390/jof6030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed A, Hassan T, Trzos-Grzybowska M, Thomas J, Quinn A, O'Sullivan M, et al. Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: A lethal combination. Med Mycol Case Rep. 2021;31:11–14. doi: 10.1016/j.mmcr.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson MD, Lewis RE, Dodds Ashley ES, Ostrosky-Zeichner L, Zaoutis T, Thompson GR, et al. Core recommendations for antifungal stewardship: a statement of the Mycoses Study Group Education and Research Consortium. J Infect Dis. 2020;222(Suppl 3):S175–S198. doi: 10.1093/infdis/jiaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verweij PE, Bruggemann RJM, Azoulay E, Bassetti M, Blot S, Buil JB, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47:819–834. doi: 10.1007/s00134-021-06449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021;73:e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 25.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gangneux J, Dannaoui E, Fekkar A, Luyt C, Botterel F, De Prost N, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 2022;10:180–190. doi: 10.1016/S2213-2600(21)00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler P, von Stillfried S, Garcia Borrega J, Fuchs F, Salmanton-Garcia J, Pult F, et al. Aspergillus tracheobronchitis in COVID-19 patients with acute respiratory distress syndrome: a cohort study. Eur Respir J. 2022;59 doi: 10.1183/13993003.03142-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ergun M, Bruggemann RJM, Alanio A, Delliere S, van Arkel A, Bentvelsen RG, et al. Aspergillus test profiles and mortality in critically ill COVID-19 patients. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.01229-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Yin C, Zhong M, Hu B, Gao X, Zhang K, et al. Incidence and outcomes of patients with COVID-19 associated pulmonary aspergillosis (CAPA) in intensive care units: a systematic review and meta-analysis of 31 cohort studies. Ann Palliat Med. 2022;11:2202–2209. doi: 10.21037/apm-21-2043. [DOI] [PubMed] [Google Scholar]
- 31.Seagle EE, Jackson BR, Lockhart SR, Georgacopoulos O, Nunnally NS, Roland J, et al. The landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis. 2022;74:802–811. doi: 10.1093/cid/ciab562. [DOI] [PubMed] [Google Scholar]
- 32.Danion F, Letscher-Bru V, Guitard J, Sitbon K, Delliere S, Angoulvant A, et al. Coronavirus disease 2019-associated mucormycosis in France: a rare but deadly complication. Open Forum Infect Dis. 2021;9:ofab566. doi: 10.1093/ofid/ofab566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Veerdonk FL, Bruggemann RJM, Vos S, De Hertogh G, Wauters J, Reijers MHE, et al. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med. 2021;9:795–802. doi: 10.1016/S2213-2600(21)00138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2021;73:e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delliere S, Dudoignon E, Voicu S, Collet M, Fodil S, Plaud B, et al. Combination of mycological criteria: a better surrogate to identify COVID-19-associated pulmonary aspergillosis patients and evaluate prognosis? J Clin Microbiol. 2022;60 doi: 10.1128/jcm.02169-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Autier B, Prattes J, White PL, Valerio M, Machado M, Price J, et al. Aspergillus lateral flow assay with digital reader for the diagnosis of COVID-19-associated pulmonary aspergillosis (CAPA): a Multicenter Study. J Clin Microbiol. 2022;60 doi: 10.1128/JCM.01689-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnacho-Montero J, Olaechea P, Alvarez-Lerma F, Alvarez-Rocha L, Blanquer J, Galvan B, et al. Epidemiology, diagnosis and treatment of fungal respiratory infections in the critically ill patient. Rev Esp Quimioter. 2013;26:173–188. [PubMed] [Google Scholar]
- 39.Seidel D, Simon M, Sprute R, Lubnow M, Evert K, Speer C, et al. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses. 2022;65:103–109. doi: 10.1111/myc.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sah SK, Shariff A, Pathakamuri N, Ramaswamy S, Ramesh M, Undela K, et al. Antifungal therapy in the management of fungal secondary infections in COVID-19 patients: A systematic review and meta-analysis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0271795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson TF, Thompson GR3, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudramurthy SM, Hoenigl M, Meis JF, Cornely OA, Muthu V, Gangneux JP, et al. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses. 2021;64:1028–1037. doi: 10.1111/myc.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Nagy E, Dobiasova S, et al. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol. 2008;46:515–521. doi: 10.1128/JCM.01915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puig-Asensio M, Ruiz-Camps I, Fernandez-Ruiz M, Aguado JM, Munoz P, Valerio M, et al. Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin Microbiol Infect. 2015;21 doi: 10.1016/j.cmi.2014.12.027. 491.e1-10. [DOI] [PubMed] [Google Scholar]
- 46.Allaw F, Kara Zahreddine N, Ibrahim A, Tannous J, Taleb H, Bizri AR, et al. First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathogens. 2021;10:157. doi: 10.3390/pathogens10020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajni E, Singh A, Tarai B, Jain K, Shankar R, Pawar K, et al. A high frequency of Candida auris blood stream infections in coronavirus disease 2019 patients admitted to intensive care units, Northwestern India: a case control study. Open Forum Infect Dis. 2021;8:ofab452. doi: 10.1093/ofid/ofab452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lestrade PPA, Meis JF, Melchers WJG, Verweij PE. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol Infect. 2019;25:799–806. doi: 10.1016/j.cmi.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Koehler P, Cornely OA, Bottiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koehler P, Cornely OA, Kochanek M. Bronchoscopy safety precautions for diagnosing COVID-19 associated pulmonary aspergillosis-A simulation study. Mycoses. 2021;64:55–59. doi: 10.1111/myc.13183. [DOI] [PubMed] [Google Scholar]
- 51.Schouten J, De Waele J, Lanckohr C, Koulenti D, Haddad N, Rizk N, et al. Antimicrobial stewardship in the ICU in COVID-19 times: the known unknowns. Int J Antimicrob Agents. 2021;58 doi: 10.1016/j.ijantimicag.2021.106409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Chang CC, Blyth CC, Chen SC, Khanina A, Morrissey CO, Roberts JA, et al. Introduction to the updated Australasian consensus guidelines for the management of invasive fungal disease and use of antifungal agents in the haematology/oncology setting, 2021. Intern Med J. 2021;51(Suppl 7):3–17. doi: 10.1111/imj.15585. [DOI] [PubMed] [Google Scholar]
- 54.Hatzl S, Reisinger AC, Posch F, Prattes J, Stradner M, Pilz S, et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: An observational study. Crit Care. 2021;25:335. doi: 10.1186/s13054-021-03753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melchers M, van Zanten AR, Heusinkveld M, Leeuwis JW, Schellaars R, Lammers HJ, et al. Nebulized amphotericin B in mechanically ventilated COVID-19 patients to prevent invasive pulmonary aspergillosis: a retrospective cohort study. Crit Care Explor. 2022;4:e0696. doi: 10.1097/CCE.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Ackerbroeck S, Rutsaert L, Roelant E, Dillen K, Wauters J, Van Regenmortel N. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care. 2021;25:298. doi: 10.1186/s13054-021-03728-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mian P, Trof RJ, Beishuizen A, Masselink JB, Cornet AD, Sportel ET. Suboptimal plasma concentrations with posaconazole suspension as prophylaxis in critically ill COVID-19 patients at risk of Covid-associated pulmonary aspergillosis. J Clin Pharm Ther. 2022;47:383–385. doi: 10.1111/jcpt.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, Mertes H, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71. doi: 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soriano MC, Narváez-Chávez G, López-Olivencia M, Fortún J, de Pablo R. Inhaled amphotericin B lipid complex for prophylaxis against COVID-19-associated invasive pulmonary aspergillosis. Intensive Care Med. 2022;48:360–361. doi: 10.1007/s00134-021-06603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoenigl M, Salmanton-Garcia J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis. 2021;21:e246–e257. doi: 10.1016/S1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]
- 61.Baracaldo-Santamaria D, Cala-Garcia JD, Medina-Rincon GJ, Rojas-Rodriguez LC, Calderon-Ospina C. Therapeutic Drug Monitoring of Antifungal Agents in Critically Ill Patients: Is There a Need for Dose Optimisation? Antibiotics (Basel) 2022;11:645. doi: 10.3390/antibiotics11050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Daele R, Wauters J, Dreesen E, Boelens J, Nulens E, Lormans P, et al. Exposure to intravenous posaconazole in critically ill patients with influenza: A pharmacokinetic analysis of the POSA-FLU study. Mycoses. 2022;65:656–660. doi: 10.1111/myc.13446. [DOI] [PubMed] [Google Scholar]
- 63.Boonstra JM, van der Elst K C, Veringa A, Jongedijk EM, Bruggemann RJ, Koster RA, et al. Pharmacokinetic properties of micafungin in critically ill patients diagnosed with invasive candidiasis. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01398-17. e01398-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roger C, Wallis SC, Muller L, Saissi G, Lipman J, Bruggemann RJ, et al. Caspofungin population pharmacokinetics in critically ill patients undergoing continuous veno-venous haemofiltration or haemodiafiltration. Clin Pharmacokinet. 2017;56:1057–1068. doi: 10.1007/s40262-016-0495-z. [DOI] [PubMed] [Google Scholar]
- 65.Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–779. doi: 10.1007/s15010-017-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: Which drug and when? Med Mycol. 2019;57(Suppl 2):S168–S178. doi: 10.1093/mmy/myy052. [DOI] [PubMed] [Google Scholar]
- 68.Jang SH, Colangelo PM, Gobburu JVS. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther. 2010;88:115–119. doi: 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 69.Douglas AP, Smibert OC, Bajel A, Halliday CL, Lavee O, McMullan B, et al. Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021. Intern Med J. 2021;51(Suppl 7):143–176. doi: 10.1111/imj.15591. [DOI] [PubMed] [Google Scholar]
- 70.Chiurlo M, Mastrangelo A, Ripa M, Scarpellini P. Invasive fungal infections in patients with COVID-19: a review on pathogenesis, epidemiology, clinical features, treatment, and outcomes. New Microbiol. 2021;44:71–83. [PubMed] [Google Scholar]
- 71.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 72.Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis. 2012;54(Suppl 1):S73–S78. doi: 10.1093/cid/cir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madhavan Y, Sai KV, Shanmugam DK, Manimaran A, Guruviah K, Mohanta YK, et al. Current treatment options for COVID-19 associated mucormycosis: present status and future perspectives. J Clin Med. 2022;11:3620. doi: 10.3390/jcm11133620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D, et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit - Florida, July-August 2020. MMWR Morb Mortal Wkly Rep. 2021;70:56–57. doi: 10.15585/mmwr.mm7002e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdolrasouli A, Armstrong-James D, Ryan L, Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses. 2017;60:758–763. doi: 10.1111/myc.12699. [DOI] [PubMed] [Google Scholar]
- 76.Ku TSN, Walraven CJ, Lee SA. Candida auris: disinfectants and implications for infection control. Front Microbiol. 2018;9:726. doi: 10.3389/fmicb.2018.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riche CVW, Cassol R, Pasqualotto AC. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J Fungi (Basel) 2020;6:286. doi: 10.3390/jof6040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macauley P, Epelbaum O. Epidemiology and mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. 2021;64:634–640. doi: 10.1111/myc.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Omrani AS, Koleri J, Ben Abid F, Daghfel J, Odaippurath T, Peediyakkal MZ, et al. Clinical characteristics and risk factors for COVID-19-associated Candidemia. Med Mycol. 2021;59:1262–1266. doi: 10.1093/mmy/myab056. [DOI] [PubMed] [Google Scholar]
- 80.Allaw F, Haddad SF, Habib N, Moukarzel P, Naji NS, Kanafani ZA, et al. COVID-19 and C. auris: a case-control study from a tertiary care center in Lebanon. Microorganisms. 2022;10:1011. doi: 10.3390/microorganisms10051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmed N, Mahmood MS, Ullah MA, Araf Y, Rahaman TI, Moin AT, et al. COVID-19-associated candidiasis: possible patho-mechanism, predisposing factors, and prevention strategies. Curr Microbiol. 2022;79:127. doi: 10.1007/s00284-022-02824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta P, Thomas M, Patel A, George R, Mathews L, Alex S, et al. Bundle approach used to achieve zero central line-associated bloodstream infections in an adult coronary intensive care unit. BMJ Open Qual. 2021;10 doi: 10.1136/bmjoq-2020-001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ture Z, Alp E. Infection control measures to prevent hospital transmission of Candida. Hosp Pract (1995) 2018;46:253–257. doi: 10.1080/21548331.2018.1510282. [DOI] [PubMed] [Google Scholar]
- 84.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis. 2014;33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 85.Kenters N, Kiernan M, Chowdhary A, Denning DW, Peman J, Saris K, et al. Control of Candida auris in healthcare institutions: Outcome of an International Society for Antimicrobial Chemotherapy expert meeting. Int J Antimicrob Agents. 2019;54:400–406. doi: 10.1016/j.ijantimicag.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 86.Centers for Diseases Control and Prevention. Infection Prevention and Control for Candida auris. Available at: Candida auris | Candida auris | Fungal Diseases | CDC. (Last accessed 30/03/23)
- 87.Piatti G, Sartini M, Cusato C, Schito AM. Colonization by Candida auris in critically ill patients: role of cutaneous and rectal localization during an outbreak. J Hosp Infect. 2022;120:85–89. doi: 10.1016/j.jhin.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Walchak RC, Buckwalter SP, Zinsmaster NM, Henn KM, Johnson KM, Koelsch JM, et al. Candida auris direct detection from surveillance swabs, blood, and urine using a laboratory-developed PCR method. J Fungi (Basel) 2020;6:224. doi: 10.3390/jof6040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, et al. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe. 2021;29 doi: 10.1016/j.chom.2020.12.002. 210-221.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rutala WA, Kanamori H, Gergen MF, Sickbert-Bennett EE, Weber DJ. Susceptibility of Candida auris and Candida albicans to 21 germicides used in healthcare facilities. Infect Control Hosp Epidemiol. 2019;40:380–382. doi: 10.1017/ice.2019.1. [DOI] [PubMed] [Google Scholar]
- 91.Johnson CJ, Eix EF, Lam BC, Wartman KM, Meudt JJ, Shanmuganayagam D, et al. Augmenting the activity of chlorhexidine for decolonization of Candida auris from porcine skin. J Fungi (Basel) 2021;7:804. doi: 10.3390/jof7100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kean R, McKloud E, Townsend EM, Sherry L, Delaney C, Jones BL, et al. The comparative efficacy of antiseptics against Candida auris biofilms. Int J Antimicrob Agents. 2018;52:673–677. doi: 10.1016/j.ijantimicag.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Public Heath England. Candida auris: laboratory investigation, management and infection prevention and control. Available at: extension://elhekieabhbkpmcefcoobjddigjcaadp/https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/637685/Updated_Candida_auris_Guidance_v2.pdf (Last accessed 30/03/23)
- 94.de Groot T, Chowdhary A, Meis JF, Voss A. Killing of Candida auris by UV-C: Importance of exposure time and distance. Mycoses. 2019;62:408–412. doi: 10.1111/myc.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shajahan A, Culp CH, Williamson B. Effects of indoor environmental parameters related to building heating, ventilation, and air conditioning systems on patients' medical outcomes: A review of scientific research on hospital buildings. Indoor Air. 2019;29:161–176. doi: 10.1111/ina.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Centers for Diseases Control and Prevention. Isolation and precautions for people with COVID-19. Available at: Infection Prevention and Control for Candida auris | Candida auris | Fungal Diseases | CDC. (Last accessed: 30/03/23)
- 97.Qian H, Zheng X. Ventilation control for airborne transmission of human exhaled bio-aerosols in buildings. J Thorac Dis. 2018;10(Suppl 19):S2295–S2304. doi: 10.21037/jtd.2018.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Humphreys H. Positive-pressure isolation and the prevention of invasive aspergillosis. What is the evidence? J Hosp Infect. 2004;56:93–100. doi: 10.1016/j.jhin.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 99.Ichai P, Saliba F, Baune P, Daoud A, Coilly A, Samuel D. Impact of negative air pressure in ICU rooms on the risk of pulmonary aspergillosis in COVID-19 patients. Crit Care. 2020;24:538. doi: 10.1186/s13054-020-03221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lindsley WG, Derk RC, Coyle JP, Martin SBJ, Mead KR, Blachere FM, et al. Efficacy of portable air cleaners and masking for reducing indoor exposure to simulated exhaled SARS-CoV-2 aerosols - United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:972–976. doi: 10.15585/mmwr.mm7027e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pampati S, Rasberry CN, McConnell L, Timpe Z, Lee S, Spencer P, et al. Ventilation improvement strategies among K-12 public schools - The National School COVID-19 Prevention Study, United States, February 14-March 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:770–775. doi: 10.15585/mmwr.mm7123e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prakash H, Singh S, Rudramurthy SM, Singh P, Mehta N, Shaw D, et al. An aero mycological analysis of Mucormycetes in indoor and outdoor environments of northern India. Med Mycol. 2020;58:118–123. doi: 10.1093/mmy/myz031. [DOI] [PubMed] [Google Scholar]
- 103.Ghosh AK, Singh R, Reddy S, Singh S, Rudramurthy SM, Kaur H, et al. Evaluation of environmental Mucorales contamination in and around the residence of COVID-19-associated mucormycosis patients. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.953750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Biswal M, Gupta P, Kanaujia R, Kaur K, Kaur H, Vyas A, et al. Evaluation of hospital environment for presence of Mucorales during COVID-19-associated mucormycosis outbreak in India - a multi-centre study. J Hosp Infect. 2022;122:173–179. doi: 10.1016/j.jhin.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shishido AA, Mathew M, Baddley JW. Overview of COVID-19-associated invasive fungal infection. Curr Fungal Infect Rep. 2022;16:87–97. doi: 10.1007/s12281-022-00434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arora U, Priyadarshi M, Katiyar V, Soneja M, Garg P, Gupta I, et al. Risk factors for Coronavirus disease-associated mucormycosis. J Infect. 2022;84:383–390. doi: 10.1016/j.jinf.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Micallef C, Ashiru-Oredope D, Hansraj S, Denning DW, Agrawal SG, Manuel RJ, et al. An investigation of antifungal stewardship programmes in England. J Med Microbiol. 2017;66:1581–1589. doi: 10.1099/jmm.0.000612. [DOI] [PubMed] [Google Scholar]
- 109.Hart E, Nguyen M, Allen M, Clark CM, Jacobs DM. A systematic review of the impact of antifungal stewardship interventions in the United States. Ann Clin Microbiol Antimicrob. 2019;18:24. doi: 10.1186/s12941-019-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ananda-Rajah MR, Slavin MA, Thursky KT. The case for antifungal stewardship. Curr Opin Infect Dis. 2012;25:107–115. doi: 10.1097/QCO.0b013e32834e0680. [DOI] [PubMed] [Google Scholar]
- 111.Khanina A, Urbancic KF, Haeusler GM, Kong DCM, Douglas AP, Tio SY, et al. Establishing essential metrics for antifungal stewardship in hospitals: the results of an international Delphi survey. J Antimicrob Chemother. 2021;76:253–262. doi: 10.1093/jac/dkaa409. [DOI] [PubMed] [Google Scholar]
- 112.Valerio M, Vena A, Rodriguez-Gonzalez CG, de Vega EC, Mateos M, Sanjurjo M, et al. Repeated antifungal use audits are essential for selecting the targets for intervention in antifungal stewardship. Eur J Clin Microbiol Infect Dis. 2018;37:1993–2000. doi: 10.1007/s10096-018-3335-2. [DOI] [PubMed] [Google Scholar]
- 113.Bienvenu AL, Argaud L, Aubrun F, Fellahi JL, Guerin C, Javouhey E, et al. A systematic review of interventions and performance measures for antifungal stewardship programmes. J Antimicrob Chemother. 2018;73:297–305. doi: 10.1093/jac/dkx388. [DOI] [PubMed] [Google Scholar]
- 114.Moni M, Sidharthan N, Sudhir S, Prabhu B, Nampoothiri V, James J, et al. A quality improvement initiative to improve the appropriateness of candidemia management by the implementation of a comprehensive candidemia care bundle at a tertiary care hospital in South India: Results of a quasi-experimental study. Medicine (Baltimore) 2022;101:e28906. doi: 10.1097/MD.0000000000028906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Markogiannakis A, Korantanis K, Gamaletsou MN, Samarkos M, Psichogiou M, Daikos G, et al. Impact of a non-compulsory antifungal stewardship program on overuse and misuse of antifungal agents in a tertiary care hospital. Int J Antimicrob Agents. 2021;57 doi: 10.1016/j.ijantimicag.2020.106255. [DOI] [PubMed] [Google Scholar]
- 116.Martson A, Alffenaar JC, Bruggemann RJ, Hope W. Precision therapy for invasive fungal diseases. J Fungi (Basel) 2021;8:18. doi: 10.3390/jof8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castelo-Branco D, Lockhart SR, Chen Y, Santos DA, Hagen F, Hawkins NJ, et al. Collateral consequences of agricultural fungicides on pathogenic yeasts: A One Health perspective to tackle azole resistance. Mycoses. 2022;65:303–311. doi: 10.1111/myc.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol. 2022;20:557–571. doi: 10.1038/s41579-022-00720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharp A, Muller-Pebody B, Charlett A, Patel B, Gorton R, Lambourne J, et al. Screening for Candida auris in patients admitted to eight intensive care units in England, 2017 to 2018. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.8.1900730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heindel J, Zweigner J, Fuchs F, Hamprecht A. Usefulness of screening for Candida auris colonisation in international patients admitted to a large university hospital. Mycoses. 2023;66:138–143. doi: 10.1111/myc.13533. [DOI] [PubMed] [Google Scholar]
- 121.Hamdy RF, Zaoutis TE, Seo SK. Antifungal stewardship considerations for adults and pediatrics. Virulence. 2017;8:658–672. doi: 10.1080/21505594.2016.1226721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whitney L, Al-Ghusein H, Glass S, Koh M, Klammer M, Ball J, et al. Effectiveness of an antifungal stewardship programme at a London teaching hospital 2010-16. J Antimicrob Chemother. 2019;74:234–241. doi: 10.1093/jac/dky389. [DOI] [PubMed] [Google Scholar]
- 123.Baddley JW, Thompson GR3, Chen SC, White PL, Johnson MD, Nguyen MH, et al. Coronavirus disease 2019-associated invasive fungal infection. Open Forum Infect Dis. 2021;8:ofab510. doi: 10.1093/ofid/ofab510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barnes RA, Gow NAR, Denning DW, May RC, Haynes K. British Society of Medical Mycology. Antifungal resistance: more research needed. Lancet. 2014;384:1427. doi: 10.1016/S0140-6736(14)61861-4. [DOI] [PubMed] [Google Scholar]
- 125.Ghosh PN, Fisher MC, Bates KA. Diagnosing emerging fungal threats: A One Health perspective. Front Genet. 2018;9:376. doi: 10.3389/fgene.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chowdhary A, Gupta N, Wurster S, Kumar R, Mohabir JT, Tatavarthy S, et al. Multimodal analysis of the COVID-19-associated mucormycosis outbreak in Delhi, India indicates the convergence of clinical and environmental risk factors. Mycoses. 2023;66(6):515–526. doi: 10.1111/myc.13578. [DOI] [PubMed] [Google Scholar]