Figure 4. Effects of persistent intrathecal 3-oxa-PD1 treatment on chronic itch and tumor growth.
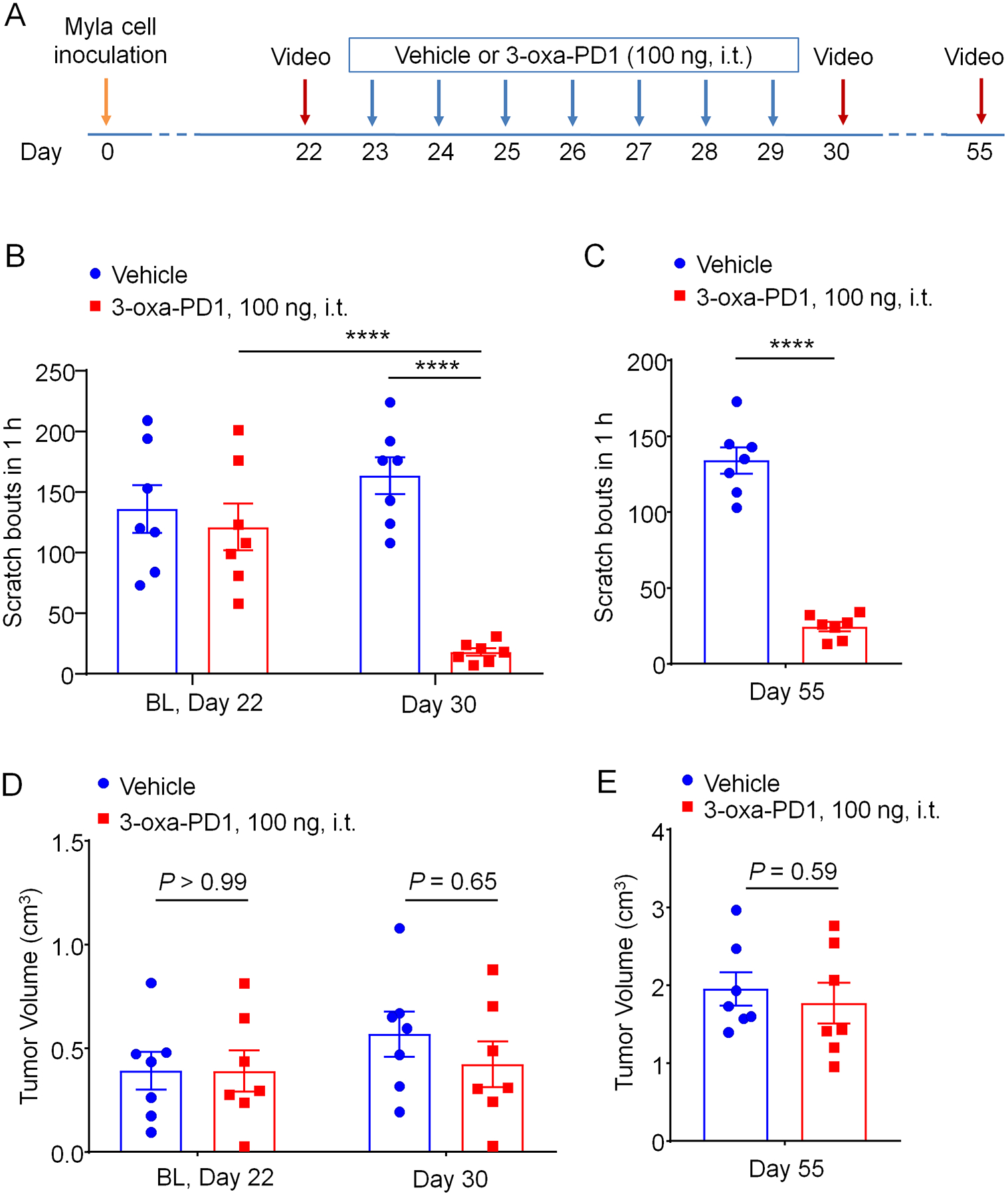
(A) Experimental paradigm of oxa-PD1 treatment and behavioral testing. Daily i.t. injection of 3-oxa-PD1 (100 ng) was given for 7 days, starting on CTCL day 23. Itching behavior was video recorded on Day 30 and Day 55. (B-C) Repeated i.t. administration of 3-oxa-PD1 substantially reduced the number of scratching in early-mid phase (Day 30, B) and late-phase (Day 55, C). n = 7 mice/group, Two-way ANOVA with Bonferroni’s post hoc test. F(1, 12) = 17.54, P < 0.0013 (B); unpaired t-test (C). (D-E) The same oxa-PD1 treatment did not affect the tumor growth in either early or late phase. n = 7 mice/group, Two-way ANOVA with Bonferroni’s post hoc test. F(1, 12) = 0.2567, P = 0.62 (D); unpaired t-test (E). Data are shown as mean ± SEM. ****P < 0.0001.
