Abstract
The metabolic actions of storage fungi and other microorganisms can cause spoilage and post-harvest losses in agricultural commodities, including flaxseed. These microbial contaminants are oxidized with hydroxyl radicals that are efficiently generated when ozone, hydrogen peroxide (H2O2) and ultraviolet (UV) light react in an advanced oxidative process (AOP). The present work explores what we believe is the first application of an AOP technology to reduce mould on whole brown and yellow flaxseed. The impact of AOP on storage and quality parameters was assessed by measuring the fatty acid value (FAV), germination rate, moisture content (MC) and visible mould growth after 12 weeks of storage at 30°C and 75% relative humidity (RH). Under these conditions, the yellow decontaminated flaxseed showed a 31% decrease in the number of seeds with visible mould without any adverse effect on germination rate, FAV and MC. In contrast, the same AOP treatment created an insignificant decrease in mould in stored brown flaxseed, at the cost of decreasing the germination rate and increasing FAV. The adverse effects of AOP on brown flaxseed were not readily apparent but became measurable after storage. Moreover, Fourier transform infrared (FTIR) spectroscopy was utilized to explore the rationale behind the different reactions of flaxseed varieties to AOP. The corresponding results indicated that the tolerance of yellow flaxseed to AOP might be related to its richness in olefins. The authors believe that technologies that harness advanced oxidative processes open new horizons in decontamination beyond ozone alone and towards increasing the shelf life of various agri-food products.
Keywords: Linseed, Cleaning, Disinfect, Sanitize, Omega-3, PUFA
Introduction
The demand for certain edible oils is expected to increase as the world’s population grows larger (Jat et al., 2019). Flaxseed (Linum usitatissimum L.) has emerged as a principal ingredient of functional foods and nutraceuticals that is of value to the global health food market. This oilseed is an excellent source of dietary fibre, vitamins, minerals and essential fatty acids, such as omega-3 and omega-6 polyunsaturated fatty acids (PUFA). Moreover, flaxseed is a rich plant source of the n-3 fatty acid α-linolenic acid (ALA) and other antioxidants. Antioxidants scavenge reactive oxygen species (ROS) and thereby alleviate oxidative stress. Dietary consumption of flaxseed can be beneficial as a part of prevention strategies for diseases where damage due to oxidative stress is implicated, such as cancer, diabetes, cardiovascular diseases and cerebrovascular stroke (Bernacchia et al., 2014; Goyal et al., 2014). Consumer demand for health-promoting products that are fresh and organic has been increasing over the past decade. However, microbial contamination is a concern in foods that are consumed raw, including flaxseed.
Non-thermal or cold methods of decontamination are thought to cause less interference with food quality attributes (e.g. flavour, essential nutrients and vitamin content) than certain thermal alternatives (Butz & Tauscher, 2002). Irradiation with ultraviolet (UV) light is an example of a cold method of decontamination that mediates oxidation by inducing the formation of free radicals and ROS. Certain ROS can act as an oxidizing disinfectant and form by-products that are non-toxic and food-safe. Specifically, ozone (O3) and hydrogen peroxide (H2O2) form oxygen gas (O2) and water (H2O), respectively. The effective use of ozone for the oxidative decontamination of cereals (Los et al., 2018), such as wheat (Raila et al., 2006; Savi et al., 2016), has been well described in the literature.
The main difference in the general biochemical composition of cereals (such as wheat) versus oilseed (such as flax) is the abundance of lipids. This difference is critical in oxidative decontamination, which is known to suffer from an ability to accelerate rancidity in fat-rich foods. Lipid oxidation can cause a loss of sensory and nutritional quality (O’Connor & O’Brien, 2006) and the formation of potentially toxic oxidation products in food (Vieira et al., 2017). It is conceivable that unwanted oxidation of hydrocarbons with double bonds (i.e. olefins) like PUFA and the omega-3 fatty acids could occur, as these compounds are labile, and radiation and/or ROS can promote their breakdown.
The synergistic combination of ozone with UV/H2O2, applied in synchrony, forms an advanced oxidative process (AOP) that can efficiently generate hydroxyl radicals and has the potential to produce results which are not possible with ozone alone (Glaze et al., 1987). Hydroxyl radicals have shorter half-lives when condensed onto a surface, compared to ozone. Hydroxyl radicals are capable of oxidizing ascorbic acid, but no such degradation of the ascorbic acid within AOP decontaminated fresh produce occurs, as the hydroxyl radicals only interact with the surface or subsurface (Fan & Song, 2020). A research gap exists regarding the application of AOPs to flaxseed, but AOPs have the potential to be adapted into approaches for decontamination.
Analysis of the impacts of ozone treatment on fatty food has involved the analysis of fatty acid through fatty acid methyl esters (FAMES) conversion (Vettraino et al., 2020), but not always an analysis of FAV as a proxy for rancidity or spoilage. Ozone treatment of antioxidant-containing blueberries showed oxidation of proteins, including enzymes involved in detoxifying ROS (Piechowiak et al., 2020). In a study of ozonated white wine grapes, Carbone and Mencarelli (2015) emphasized that oxidative stress of the fruit can be positive, in that it triggers defensive mechanisms such as the production of compounds that are healthy for humans, such as stilbenes and polyphenols. However, a review by Gayán et al. (2014) concluded that combinations of UV with oxidant compounds have the potential to enhance the safety and stability of processed food.
Shen and Singh (2022) performed an AOP study on another oilseed, peanut, without ozone and have described how previous studies using H2O2 or UV light in isolation have demonstrated shortcomings, such as long treatment time. Compared to peanuts, flaxseeds have increased surface-to-volume ratio and hygroscopic mucilage that causes grains to cake when wet with liquid. This makes it essential to regulate H2O2, by using a low volume and/or by better aerosolizing it to create humidity rather than large droplets. Our addition of a dry gas phase, ozone, to the AOP does not risk increased liquid moisture absorption into the seed.
We hypothesize that brief exposure to an AOP employing a combination of ozone and UV/H2O2 could decontaminate the surface of flaxseed while not oxidizing the lipids within the seed, due to the shorter half-life of the hydroxyl radicals compared to ozone. The present study examined this hypothesis, through the first advanced oxidative decontamination of flaxseed processed with a unit that provides closed-loop control of ozone, H2O2 and irradiation with UV light. The focus of this study is the advanced oxidative decontamination technique that could be very effective for high oil content seeds, in particular flax. Quality-determining tests were conducted for the fatty acid value (FAV), germination rate and visible mould growth on saltwater filter paper (SFP). We tested the quality of flaxseed after decontamination, using applicable classical methods of grain analysis typically used at commodity handling and storage facilities. Additionally, FTIR spectroscopy was performed to assess the levels of olefins in the flaxseed, relative to the total methyl/methylene long-chain lipid content. Our infrared spectroscopic methods take advantage of strong and nascent signals stemming from the vibrations of unsaturated hydrocarbons.
Materials and Methods
Sample Preparation
Mixed varieties (CDC Bethune and Glas) of brown flaxseed and the VT-50 variety of yellow flaxseed (Fig. 1) were chosen for their reportedly contrasting levels of ALA. The samples of flax used in this study were procured from a local supplier (Johnson Seeds Ltd, Arborg, Manitoba) in August 2021 and stored at 4°C in polyethylene bags. The moisture content (MC) of samples was measured in triplicate using the standard hot air oven procedure (ASABE, 2012) and expressed on a wet basis. This measurement was done: (1) upon receiving samples; (2) after conditioning; (3) after decontamination; and (4) weekly during storage.
Fig. 1.

Photomicrograph of A brown and B yellow flaxseed
Refrigerated flaxseed samples were conditioned as per the method of Mundhada et al. (2022) to obtain an MC that matched conditions that promote rapid microbial growth and spoilage (i.e. 30°C and 75% RH). The flaxseed was equilibrated at 4°C for 1 week in sealed double bags after the addition of a calculated volume of sterile water to allow the absorption and redistribution of water. No more than 2% (w/w) of sterile water was added at a time to prevent caking of the grains. After conditioning, the final MC of 9.2 ± 0.2% (w/w) was achieved for both the yellow and brown flax, as verified by MC testing. Control flaxseed was subdivided from the conditioned flax and not decontaminated, while other conditioned flaxseed was decontaminated (Fig. 2).
Fig. 2.
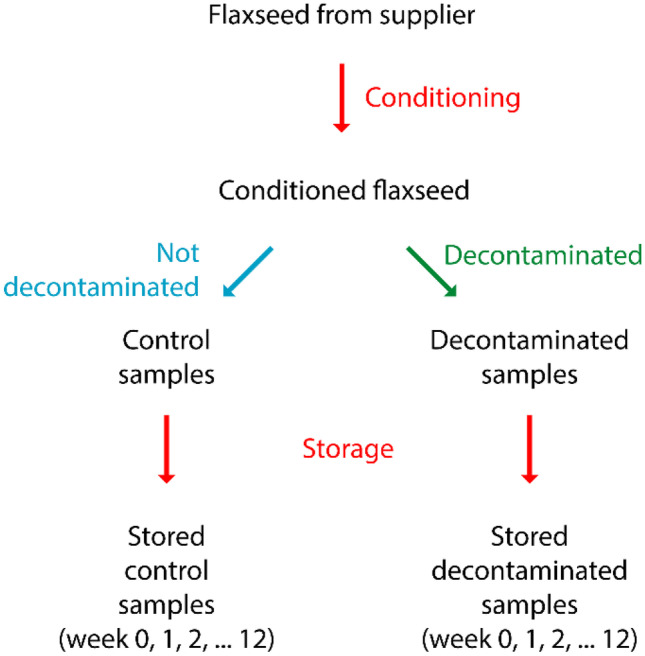
Flow chart of flaxseed samples
Decontamination
Decontamination of conditioned flaxseed samples was carried out using a Clēan Flow Health Care Mini (Clēan Works, St. Catharines, ON). A mass of 500 g of the seed was spread over aluminium foil covering the surface area of a plastic tray (0.71 g/cm2), creating a multi-kernel layer. The built-in conveyor belt of the Clēan Flow unit was set to 41% of maximum speed to intake the trays at a continuous rate (5 trays per 30 s). The sample trays were irradiated with UV light (10 UV-C bulbs, > 0.8 mW/cm2) with a simultaneous spray of 3% H2O2 Clēan Flow solution (20 mL/min flow speed) in the presence of ozone (3–4 ppm). The flaxseed-laden trays exited the treatment chamber after an exposure time of 30 s. The trays were loosely covered with overhanging alcohol-disinfected aluminium foil and allowed to equilibrate at ambient conditions for 10 min to evaporate any residual surface moisture.
Storage
The storage was performed for control and decontaminated flaxseed in triplicate for each colour (yellow and brown). The flaxseeds were stored for 12 weeks at 30°C and 75% RH. The storage procedures are adapted from similar relevant works (Mundhada et al., 2022; Sravanthi et al., 2013). The surfaces of the storage setup (Fig. 3) were alcohol disinfected with 70% ethanol prior to use.
Fig. 3.
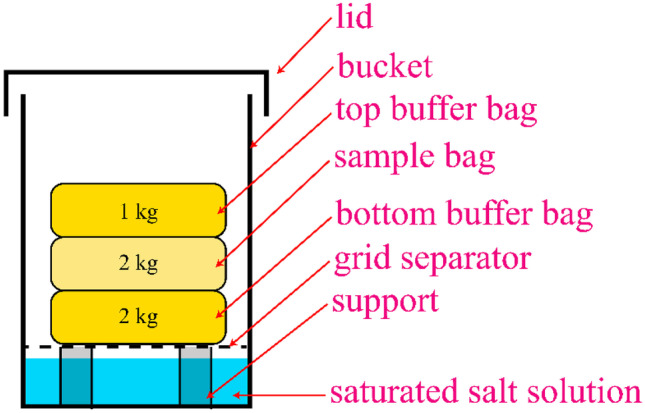
Diagram of the storage setup
To summarize, storage occurred in a Conviron growth chamber (Model E15, Controlled Environments Inc., Winnipeg, MB) equipped with controllers (CMP4030 series) set to the selected temperature and RH. The RH was buffered with a 2 L slurry of saturated NaCl in 5-gallon plastic buckets. PVC tubing supports and a plastic grid separator plate were placed in the bucket. The bucket openings were covered loosely with overhanging aluminium foil. Unlike a lid with a gap, this facilitates oxygen diffusion into the system while discouraging the influx of spores or airborne microbes. HOBO data loggers (Onset Computer Corp. Bourne, MA) in the buckets and environmental chamber captured temperature and humidity readings at 15-min intervals for the duration of storage.
Mesh sample bags were laundered with household bleach before being filled with 2 kg of either the control or decontaminated flaxseed. Mesh buffer bags were filled with 1 kg of flaxseed that had not been decontaminated. The buffer bags were placed above and below the sample bag in the buckets to buffer moisture loss/gain and to promote decay during storage.
At weekly intervals, the sample bags were mixed, and approximately 200 g of flaxseed was scooped out. The weekly samples of the stored flaxseed underwent germination and SFP testing within 48 h of removal from the storage setup. The remaining portion of the weekly sample was vacuum-packed and kept frozen (−18°C) until FAV testing and FTIR spectroscopy was performed.
Quality Evaluation
Germination Testing
A filter paper (90 mm, Whatman 3) was added to a Petri dish (90 mm, gamma-irradiation sterilized, Fischer Scientific) and wetted with 5.5 mL of deionized sterile water. Twenty-five seeds were placed on the wet paper and incubated for 7 days at 20 ± 2◦C. The number of seeds that showed signs of germination, defined by a minimum of 2 mm length radical emergence, was counted and expressed as a percentage (Nadimi et al., 2022a, c). The germination testing was performed twice for each weekly sample of seeds from the triplicate storage buckets (n = 6).
Saltwater Filter Paper Testing
The SFP testing procedure was identical to the germination testing procedure, except that instead of water, 5.5 mL of 7.5% (w/w) saltwater was added to wet the filter paper and prevent germination (Mills et al., 1978). After incubation, the number of seeds without visible mould growth detectable with the naked eye or dissecting microscope was counted in each dish. The number of seeds without visible mould growth was expressed as a percentage.
Fat Extraction
Triplicate weekly samples of stored flaxseed (n = 3) were thawed and oven-dried (model ON-11E; JeioTech, Korea) for 48 h at 50°C (Piva et al., 2018). The flaxseed samples were then ground using a Stein laboratory mill (Model M-2, Fred Stein Laboratories Inc., Atchison, KS) for a duration of 1 min.
The lipid portion of the seed was separated with a Goldfisch Fat Extractor (Laboratory Construction Co., Kansas City, MO). An analytical balance (Sartorius Quintix 313–15, Cole-Parmer) was used to weigh out 5 g of ground flaxseed. Hexane was percolated through the ground flaxseed for 16 h without the aid of a shaker and then evaporated using built-in heating mantles to leave the oily extract.
Additional steps were added to the fat extraction procedure to ensure accurate percent masses of lipids were measured for the yellow and brown flaxseed. The glassware was baked at 125 °C for 1 h prior to fat extraction and then cooled for 1 h. Further evaporation of the extracted oil was performed in an oven at 100°C for 1 h before cooling. All cooling to room temperature took place in a desiccator prior to weighing with the abovementioned analytical balance.
FAV Testing
A 0.04% phenolphthalein indicator was added to a solution of 50% toluene and 50% absolute ethanol. Extracted oil dissolved in 25 mL of the indicator solution was titrated with a standardized KOH base solution. The fatty acid value (FAV) was then calculated as milligrammes of KOH required to neutralize the free fatty acid (FFA) in 100 g of dry grain (Mundhada et al., 2022).
FTIR Spectroscopy
In preparation for transmission FTIR imaging, control yellow and brown flaxseed was soaked in water and embedded in an optimal cutting temperature compound (OCT, Tissue-Tek, Sakura Finetechnical Co. Tokyo, Japan). The samples were cryo-sectioned to 10 µm thickness in a cryostat (Cryotome E; Thermo Fisher Scientific, Waltham, MA) with Shandon PTFE-coated blades (MX-35 low profile, Thermo Scientific). The cryosections were mounted on BaF2 windows (25 mm diameter, 1 mm thickness, Knight Optical, Harrietsham, Maidstone, UK). Each window was stored in a sealed 1-inch plastic wafer carrier box on dry ice. The cryosection-laden windows were thawed and dried in dark room temperature conditions and immediately imaged.
Transmission FTIR images were acquired in triplicate with an Agilent Cary 670/620 FTIR micro-spectrometer (Agilent Technologies, Santa Clara, CA) equipped with a Focal Plane Array (FPA, 64 × 64 pixels, MCT detectors) with a high magnification upgrade (1.1 µm pixel edge). The spectral resolution was 4 cm−1, and 256 scans were co-added. Each pixel spectrum of the FTIR images was processed. An area where the tissue was shredded was blacked out in the image for clarity as per the method of Stitt et al. (2012).
In preparation for attenuated total reflectance (ATR)-FTIR spectroscopic measurement, the whole flaxseed was ground for 60 s with the Stein laboratory mill. Handheld ATR-FTIR spectra of the ground flaxseed were recorded with an Agilent 4300 handheld FTIR spectrometer (Agilent Technologies, Santa Clara, CA) equipped with a diamond ATR accessory. The spectral resolution was 4 cm−1, and 64 scans were co-added. Spectra were acquired by pressing the diamond tip to each of the triplicate (n = 3) ground flaxseed samples.
The transmission FTIR and ATR-FTIR data were analysed with Agilent Resolutions Pro and MATLAB 2022b software, respectively. The processing procedure used in this study was previously explained by Stitt et al. (2012). In summary, the area under the curve of the FTIR spectra was calculated as the baseline-subtracted integration of the intensity of the olefinic peak centred at 3012 cm−1 (peak width: 3001–3019 cm−1; baseline: 2989–3030 cm−1) and the symmetric and asymmetric CH2 CH3 stretching region (peak width: 2844–2857 cm−1; baseline: 2795–3001 cm−1). The baseline-subtracted area of the 3012 cm−1 spectral peak was divided by that of the CH2 CH3 stretching region for each spectrum to form a ratio. For FTIR images, the resulting ratio of areas for each pixel spectrum was colour-scaled to a heatmap (blue and red representing low and high values, respectively).
Statistical analysis was conducted using MATLAB software. The heterogeneity of variances was assessed with Lavene’s test, and normality was investigated with the Shapiro-Wilk test. The two-sample t test was performed for data with normal distributions with equal variances based on the results of former and latter testing, respectively. Further alternative t testing was applied appropriately if the assumption of equal variances was violated. Where normality and variance assumptions for t tests were not satisfied, the Mann-Whitney U test was performed. The threshold suggesting significance was set at p = 0.05, and probability values greater than that were interpreted as non-significant effects. The independent variables tested were exposure to the advanced oxidative process or the colour of our flaxseed, while the dependent variables were the measured quality parameters.
Results and Discussion
Moisture Content Testing
The initial MC of brown and yellow flaxseed was 6.5 ± 0.1% and 7.0 ± 0.2% (w/w), respectively. The abovementioned MCs are considered low and are consistent with the hot dry 2021 Manitoba growing season. Conditioning took 2 weeks to increase the MC of the flaxseed to a level of 9.2 ± 0.2% (w/w).
In the Clēan Flow unit, the moistening action of the H2O2 spray seemed to counteract the drying effect of the fanned ozone for the brown flax. As a net result, the mean of the MC of the brown control flaxseed was not significantly different from that of the brown decontaminated flaxseed (Table 1). However, for the yellow flaxseed, there was a loss of MC to a value of 8.6 ± 0.1% after decontamination.
Table 1.
Moisture content of brown (CDC Glas and Bethune) and yellow (VT-50) flaxseed
| Moisture content (%) | ||||
|---|---|---|---|---|
| Brown | Yellow | |||
| Mean | SD | Mean | SD | |
| Flaxseed from supplier | 6.5 | 0.1 | 7.0 | 0.2 |
| Control samples | 9.2 | 0.2 | 9.2 | 0.2 |
| Decontaminated samples | 8.7 | 0.6 | 8.6 | 0.1 |
| Stored control samples | 9.1 | 0.2 | 8.8 | 0.3 |
| Stored decontaminated samples | 9.1 | 0.3 | 8.6 | 0.2 |
The movement of gas, in the form of ambient air, through the grain, i.e. aeration, is commonly used in modern agriculture to lower MC. However, excessively fanning/blowing overly dry gasses of any kind, including ozone, over grain would have the risk of reducing the MC to a point where the seed becomes brittle and susceptible to mechanical breakage. Considering researchers have recently reported that MCs at or below 6% for flaxseed can make the seeds susceptible to mechanical damage (Nadimi et al., 2022b), the MC of 8.6% should not have any of such concerns. Considering very high MCs can also lead to spoilage, keeping the seeds at a safe MC is critical.
Based on the above discussions, drying grain during aeration with ozone or during advanced oxidative decontamination could be advantageous to storage, as lower MC is associated with a slower spoilage rate. Conversely, spraying excessive amounts of liquid H2O2 on the hygroscopic grain would have the risk of increasing grain MC, thereby accelerating spoilage.
During the subsequent 12 weeks of storage, the average weekly MCs of the control and decontaminated brown flaxseed were 9.1 ± 0.2 and 9.1 ± 0.3%, respectively, which were not significantly different. The average weekly MCs of the stored yellow control (8.8 ± 0.3%) and decontaminated flaxseed (8.6 ± 0.2%) were also not significantly different from each other. The recorded MCs indicate that it is likely that the equilibrium MC of the yellow variety is lower than that of the brown at our chosen temperature and RH.
Germination Testing
The germination rate of the brown and yellow flaxseed was initially 99 ± 2 and 97 ± 5%, indicating good seed quality. This result is consistent with the short lag time between harvesting and testing and good handling practices by the supplier. The advanced oxidative decontamination performed on the flaxseed with the Clēan Flow unit did not significantly lower the germination rate immediately. After decontamination, the germination rate was measured to be 96 ± 4 and 88 ± 4% for brown and yellow flaxseed, respectively. The decrease in the germination of the yellow flaxseed from 97 to 88% was determined to be insignificant after further statistical testing, as the Mann–Whitney test was not passed at a threshold of p = 0.05.
During storage, the germination rate decreased from its initial high value for both seed colours, including control and decontaminated flaxseed (Fig. 4). At the 12-week time point at the end of the storage study, the brown control flaxseed had a germination rate of 80 ± 3%, whereas the brown decontaminated flaxseed had a germination rate of 65 ± 6%. This significant difference indicates some damage to the viability of brown flaxseeds that only becomes apparent after the passage of time and storage. In contrast, the 12-week stored yellow flaxseed did not have a significant difference in the germination rate between the control (61 ± 15%) and decontaminated flaxseed (63 ± 10%).
Fig. 4.
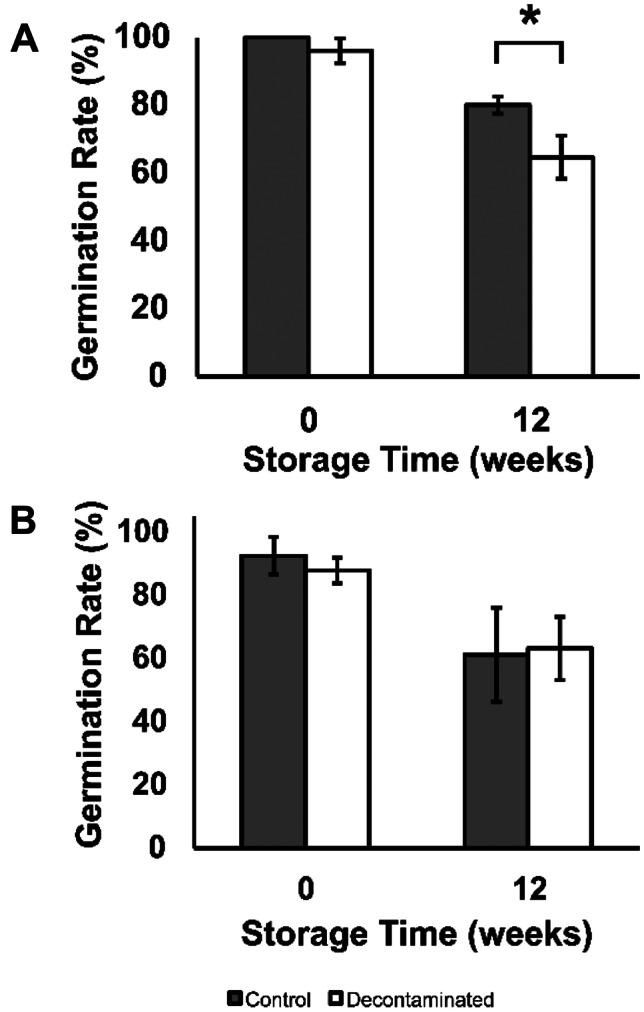
The germination rates of A brown and B yellow flaxseed and (grey bars) control and (white bars) decontaminated samples during storage. Asterisk indicates a significant difference between the control and treatment (two-sample t test; *p ≤ 0.05)
The living zygote within a seed is vulnerable and is known to be damaged by overly high concentrations of ozone (Pandiselvam et al., 2020). Oxidative stress is implicated in many human diseases but is also relevant to seed germination. Germination involves rapidly dividing cells with an intense metabolic activity that produces ROS. Omega-3 fatty acid antioxidant properties neutralize oxidative stress, the imbalance between the production and detoxification of ROS. Low levels of ALA in flaxseed of certain genetic backgrounds have been related to a decline in seed vigour in stressed conditions (Saeidi & Rowland, 1999). Oxidative decontamination could be considered a stressful condition for the flaxseed, and the different seed vigour/germination rate responses observed in the flaxseed varieties/colours may be related to their ALA level.
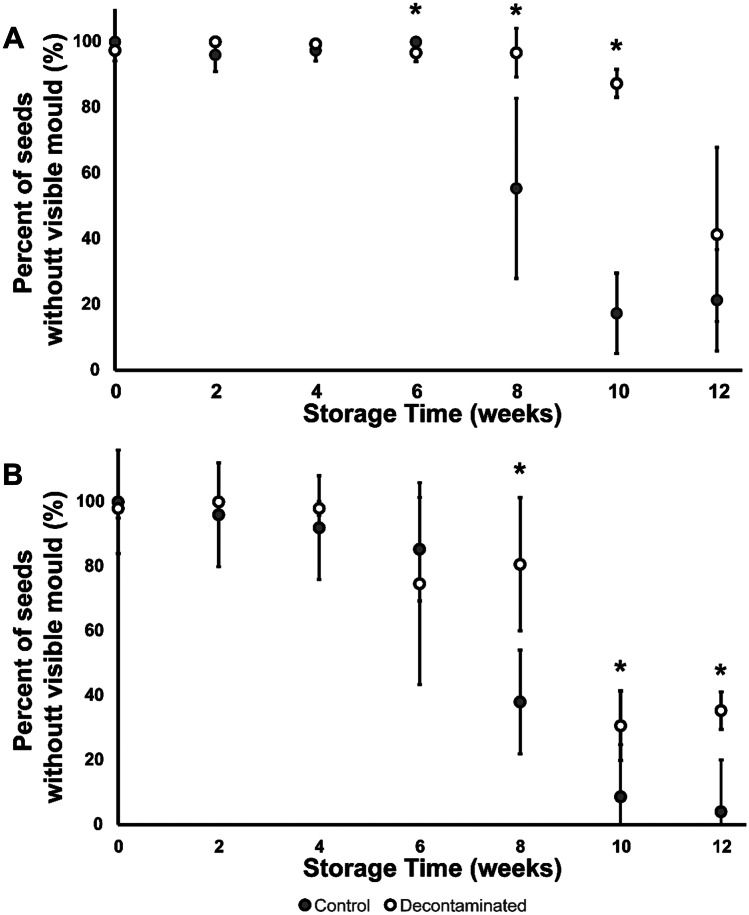
Saltwater Filter Paper Testing
SFP testing showed that the percentage of seeds without visible mould was at or near 100% at the beginning of the storage study (Fig. 5). The percentages decreased over the course of the storage study. Twelve weeks of storage were a sufficient time for the visible mould percentage of the control yellow flaxseed to drop completely to a value that is not significantly different from 0% (i.e. 4 ± 4%). Differences between the control and decontaminated flaxseed became apparent only after 8 weeks of storage. The decontaminated flaxseed showed higher percentages of seeds without visible mould growth for both colours.
Fig. 5.
Saltwater filter paper (SFP) results for the stored A brown and B yellow flaxseed. Data points are plotted in grey and white for control and decontaminated samples, respectively. Asterisks indicate a significant difference between the control and decontaminated (two-sample t test; *p ≤ 0.05)
Our observation at the 12th-week time point at the end of the storage indicates a significantly higher percentage of seeds without visible mould for decontaminated yellow flaxseed compared to the control. The brown samples also decreased the percentage of seeds with visible mould growth after 12 weeks of storage, but the difference was insignificant at p ≤ 0.05.
Prior works on the application of the Clēan Flow unit to decontaminate N95 respirators during the COVID-19 pandemic established that the unit is capable of efficiently sterilizing viral and bacterial burdens (Clark et al., 2021). The present study extends the use of the same effectors (i.e. UV light, ozone and H2O2) to demonstrate the grain decontamination abilities of the Clēan Flow. However, there is a need to further study the various strains of grain-afflicting moulds. As well, there is a research gap in the application of advanced oxidative decontamination techniques to inactivate mycotoxins.
FAV Testing
The FAV was not significantly affected by decontamination at 0 weeks of storage for both colours (Fig. 6). After 12 weeks of storage, there was an increase in the FAV of the brown decontaminated flaxseed compared to the control. This observation indicates that the fatty acid contents of the seed were degraded faster in the brown seed after the advanced oxidative decontamination. On the other hand, no significant changes were observed in the yellow variety after 12 weeks.
Fig. 6.
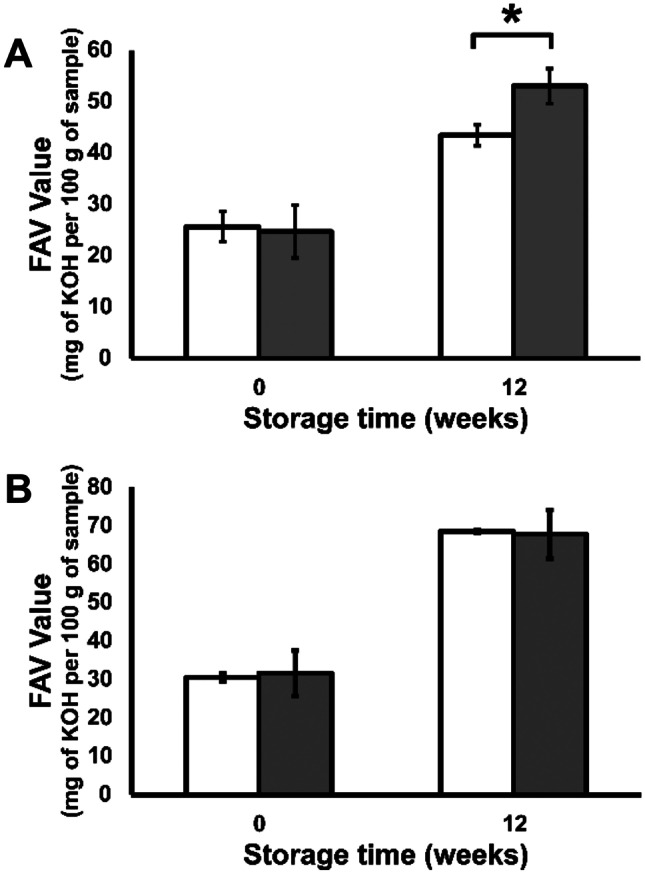
Fatty acid value of A brown and B yellow flaxseed (grey bars) and control and (white bars) decontaminated samples during storage. Asterisk indicates a significant difference between the control and decontaminated (two-sample t test; *p ≤ 0.05)
Interestingly, our results are the opposite of what was reported in a study of ozone fumigation of flaxseed by Bechlin et al. (2019), where ozone fumigation caused an increase in the FFA increased in a yellow, but not in a brown variety. This inconsistency could be due to several differences between the experimental design of the two studies. For example, herein, we used unpackaged flaxseeds under advanced oxidative decontamination with ozone concentrations of 3–4 ppm for 30 s. In the other study, packaged flaxseeds were utilized with ozone fumigation at a concentration of 10 ppm for 120 min. The increase in FFA in response to ozone exposure is problematic as it indicates that the lipid contents of the seeds were oxidized and that rancidity was accelerated. Concerningly, the increase in rancidity was not apparent immediately after the oxidative treatment but rather as a delayed effect that only became measurable with the passage of time. Our literature review has indicated that storage studies are necessary for assessing the full-/long-term consequences of oxidative decontamination on lipid-rich commodities.
Percent Mass of Lipid and Mass of Flaxseeds
The control brown flaxseed studied was 36 ± 3% (w/w) oil. The yellow flaxseed (VT-50) was 44 ± 1% w/w oil. It is reported that the VT-50 variety has a higher 67.8% ALA content as a percent of total fatty acid composition. In contrast, the brown CDC Bethune and Glas 2012 varieties have ALA percentages of 54.2 and 58.4%, respectively (You et al., 2016). It follows that the yellow flaxseed studied here should have a higher percentage of ALA per mass dry weight of seed than the brown.
Genetically purebred varieties are generally not commercially available. Blended brown flaxseed is typical of what is commercially traded and available to food processors, manufacturers and retail customers. The brown genetically blended sample we employed was sufficient for the purpose of illustrating a disparity in ALA content. How that disparity may produce significantly different FTIR spectroscopic signatures for each flax is explored in the next section.
FTIR Spectroscopy
Transmission FTIR Imaging
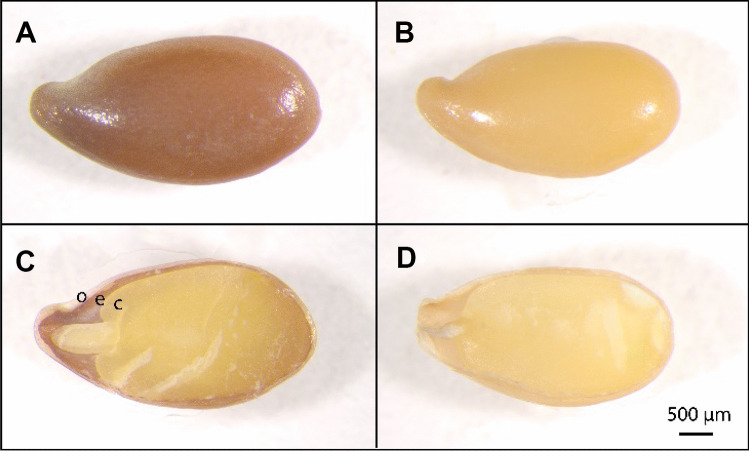
The internal anatomical features of the flaxseed can be seen when the seed is cut in either the longitudinal (Fig. 7) or transverse (Fig. 8A) direction. The flaxseed was cryo-sectioned in the transverse direction after soaking and embedding (Fig. 8B), but the tissue was prone to tearing/separating at the mechanically weak region between the endospermic and the cotyledonous tissue.
Fig. 7.
Photomicrographs of (top) A, B intact and (bottom) C, D longitudinally bisected (left) A, C brown and (right) B, D yellow flaxseed. Lowercase letters indicate anatomical features (o) the outer hull or seedcoat, (e) the endosperm and (c) the cotyledon
Fig. 8.
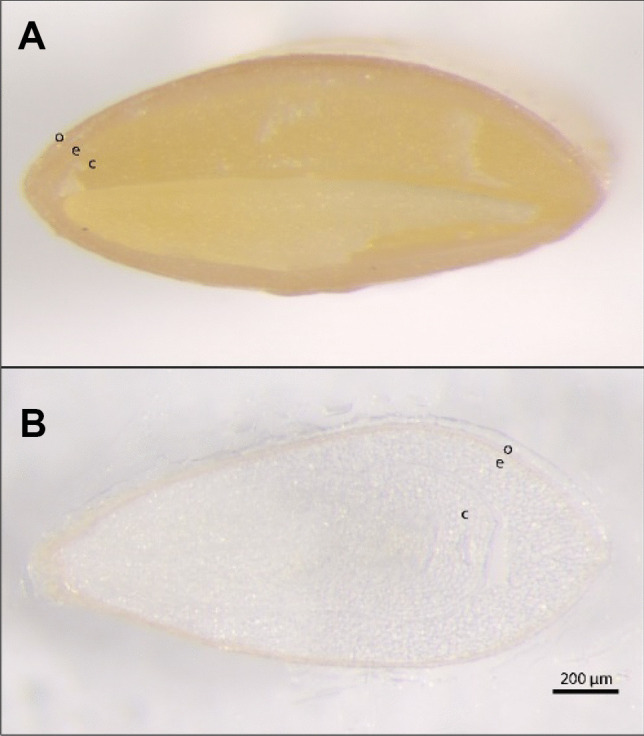
Visible photoimages of transversely cut flaxseed. (Top) A Bisected and (bottom) B cryo-sectioned. (o) The outer hull, (e) the endosperm and (c) the cotyledon
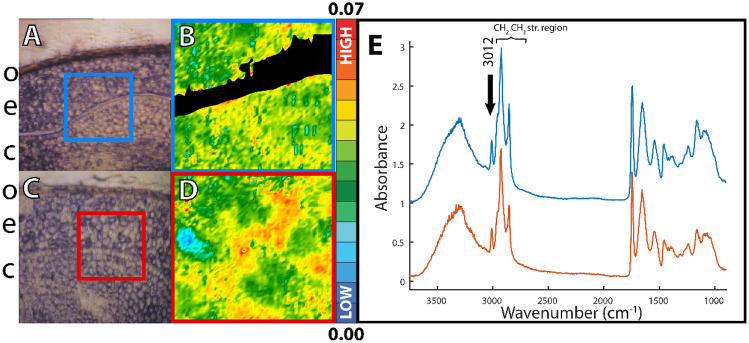
Swaths of tissues were intact enough to acquire transmission-mode FTIR hyperspectral images, as seen in Fig. 9. The blacked out area of the image in Fig. 9B had low or no amide I and II bands in the FTIR spectra, indicating no protein and a lack of tissue where a rip occurred. Examining a ratio, rather than the peak alone, was used as a strategy to deemphasize changes to spectral peaks that are a consequence of a longer/shorter path length caused by uneven, thicker tissue sectioning. The FTIR images of the brown flaxseed had pixel spectra with moderate calculated ratios (green and yellow pixels Fig. 9B) of two spectral features: (1) the area under the peak at 3012 cm−1 and (2) the area under the total CH2 CH3 stretching region between 2844 and 2857 cm−1. For the yellow flaxseed (Fig. 9C), the FTIR images have more pixel spectra with a higher calculated ratio (red pixels, Fig. 9D). An increase in the ratio of the area under the olefinic peak at 3012 cm−1 to the CH2 CH3 stretching region area was only subtly apparent in the unintegrated FTIR spectra and was not present in all pixel spectra (Fig. 9E).
Fig. 9.
Transmission-mode FTIR imaging of (top) A, B brown and (bottom) C, D yellow flaxseed. A, C Visible photoimages of cryosections of the flaxseed showing (o) the outer hull, (e) the endosperm and (c) the cotyledon. The blue and red boxes are 70.4 µm wide and indicate the areas imaged. B, D False-colour FTIR images processed for the ratio of two areas under the curve of the FTIR spectra, mapped to a common colour scale. The black pixels indicate an area with a tear in the tissue. E Selected FTIR spectra from the images are displayed on a common scale, offset for clarity
The spectra of the flaxseed have a relatively larger absorbance between 1200 and 900 cm−1 compared to the mammalian tissue that is rich in PUFA reported by Stitt et al. (2012). Absorbance in this spectral region could be due to the presence of C-O-H and C-O-C bending and the C-O and C-C stretching of carbohydrates (Wiercigroch et al., 2017). Absorption in the range of 1690– 1727 cm−1 is also substantial, and this is consistent with the presence of carbonyl (C=O) associated with FFA, which has been studied in flaxseed oils (Schönemann & Edwards, 2011).
Overall, the transmission FTIR micro-spectroscopy of the flaxseed was challenging, and due to the poor quality of the tissue, only the control flaxseed could be analysed. In thin layers, flaxseed oil can oxidize in as little as 5 h in ambient conditions (Van der Weerd et al., 2005). The thinness of the cryosections makes them vulnerable to oxidation due to ambient atmospheric oxygen during sectioning and imaging. One possible solution to the problem of tissue quality would be to dissect and remove the seed coat prior to sectioning. The seed coat readily separates after the seed is transversely bisected. However, we did not attempt imaging without the seed coat, due to the relevance of the seed coat to surface decontamination.
Handheld ATR-FTIR
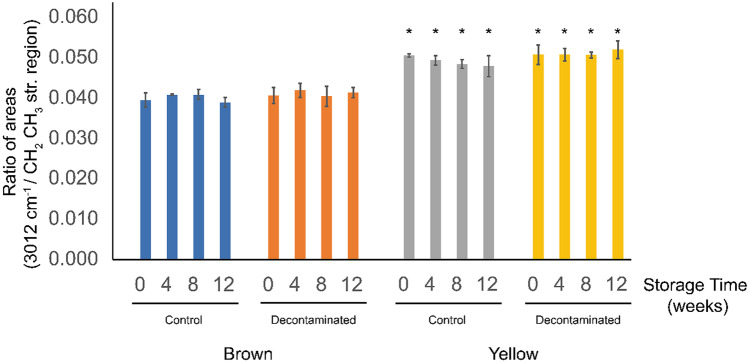
For the handheld ATR-FTIR spectra of ground flaxseed, it was observed that the yellow and brown flaxseed had a consistent difference in the areas of the peak 3012 cm−1 relative to the area of the CH2 CH3 stretching region (Fig. 10). After the handheld ATR-FTIR spectra were processed, it became apparent that all the stored yellow flaxseed spectra had a higher peak ratio than the stored brown flaxseed spectra (Fig. 11). The intensity of the olefinic 3012 cm−1 peak relative to the CH2 CH3 stretching region did not decrease significantly over time during the storage study or due to decontamination.
Fig. 10.
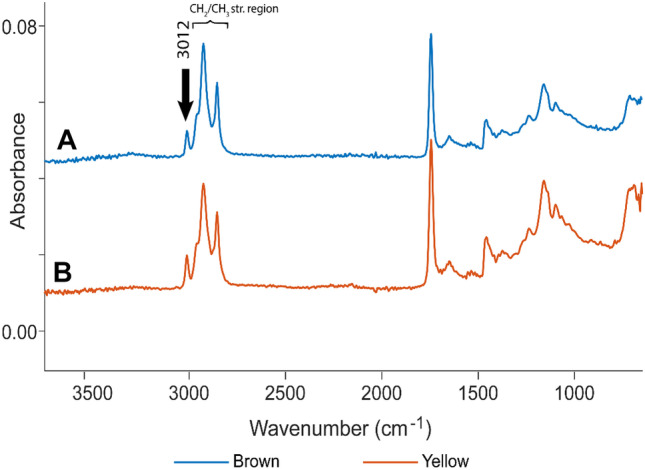
Representative handheld ATR-FTIR spectra of A brown and B yellow flaxseed from the storage study. Spectra are on a common scale, offset for clarity
Fig. 11.
Results of ratioing the area of the olefinic 3012 cm−1 peak to the CH2 CH3 stretching region of handheld ATR-FTIR spectra of stored flaxseed. Asterisks indicate a significant difference (p ≤ 0.05) between brown (CDC Bethune and Glas) and yellow (VT-50) varieties
Van der Weerd et al. (2005) observed a rapid (5 h) decrease in the olefinic peaks in thin films of flaxseed oils. Our results on the stability of the olefinic peaks over the longer term (3 months) may be explained by the fact that the studied flaxseed tissue structure is intact during storage. Thin layers, such as cryosections or films, have a higher surface area exposed. The intact tissue and the outer hull would also offer some protection from oxygen.
The sample preparation and analysis involved with acquiring handheld ATR-FTIR spectra of ground flaxseed were simple and effectively showed spectral contrast between yellow and brown flaxseed. Hence, our experiment suggests that ATR-FTIR is a faster tool than transmission FTIR for studying the seed as a whole, when tissue-specific changes are not required and when exposure to ambient oxygen is an issue and data must be acquired quickly.
The higher percentage of ALA and other unsaturated compounds in the yellow flaxseed relative to the amount of long-chain fatty acids explains the higher ratio of areas (3012 cm−1/CH2 CH3 stretching region) observed in the FTIR spectra. These unsaturated compounds may be relevant to reducing damage to quality. A study by Mohanan et al. (2018) found that some natural antioxidants in flaxseed oil have an ability to prevent lipid oxidation that is on par with synthetic antioxidants. It may be possible that the increased presence of antioxidants in the yellow flaxseed counteracts lipid oxidation and oxidative damage due to oxidative decontamination, preventing the promotion of oxidative rancidity during storage. There could be a more complex relationship here, as biological systems that are rich in olefins often have other antioxidant systems that protect them from lipid peroxidation. It may not be the unsaturated lipids acting directly to detoxify oxygen but rather related systems at work.
Conclusions and Future Work
The potential of advanced oxidative decontamination consisting of ozone, H2O2 and UV treatment was explored for two varieties of flaxseeds, viz. brown and yellow. Our applied treatments on yellow flaxseed were successful in two respects: (1) mould reduction and (2) maintenance of quality parameters such as germination rate and FAV. On the other hand, the same treatment on brown flaxseed diminished its quality without significantly improving mould reduction. The observed differences in the behaviour of decontaminated flaxseed varieties over time reveal the need for storage/shelf life studies to elucidate the full impacts of ozone and ozone-related processes.
Different reactions to decontamination treatments for flaxseed varieties may be due to differences in their compositional constitution, e.g. amounts of ALA. Our analysis indicates that the yellow and brown flaxseed had FTIR spectra that were distinguishable from each other using the relative strength of two features, specifically the olefinic C-H stretching ratioed to the symmetric and asymmetric C-H stretching region (C=C-H / CH2 CH3). The ALA content disparity between varieties studied produced significantly different FTIR spectroscopic signatures for each of our colour samples of flax. The increased resistance of yellow flaxseed to oxidation could be related to the rich presence of lipid double bonds.
Adjustments of decontamination parameters (e.g. exposure time, irradiation intensity and concentrations) could reduce or eliminate the negative impacts on the brown variety and increase the microbial reduction of yellow seed. Indeed, the impacts of advanced oxidative decontamination are different for different varieties/colours of flaxseed. Our study suggests that flaxseed varieties with high levels of antioxidants, e.g. the yellow flaxseed variety studied here, are resistant to deterioration due to oxidation and can withstand the more aggressive treatment. Overall, the authors believe an intense, but short and calibrated exposure to a dosed combination of three decontaminating agents (i.e. ozone, H2O2 and UV) is a promising tool to decontaminate flaxseeds without inducing adverse side effects on quality.
Acknowledgements
The authors would like to thank Brian Johnson and Johnson Seeds Ltd. for the donation of flaxseed, Alex Ko for the resourcing of the Clēan Flow Unit and Sristi Mundhada for her technical assistance.
Author Contribution
Catherine R Findlay: methodology, software, validation, data curation, formal analysis, investigation, visualization and roles/writing, original draft. Jaskirat Singh: investigation. Mohammad Nadimi: writing, review and editing. Jitendra Paliwal: conceptualization, funding acquisition, project administration, supervision, resources and writing, review and editing.
Funding
This work was supported by Mitacs through the Mitacs Accelerate programme. The authors received support from the National Research Council Canada’s Pandemic Research Challenge Program in the form of a Clēan Flow Health Care Mini Unit provided to the University of Manitoba.
Data Availability
The authors can confirm that all relevant data are included in the article and/or its supplementary information files.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Catherine RJ Findlay, Email: catherine.findlay@umanitoba.ca.
Jaskirat Singh, Email: singh.jaskirat1033@gmail.com.
Mohammad Nadimi, Email: mohammad.nadimi@umanitoba.ca.
Jitendra Paliwal, Email: j.paliwal@umanitoba.ca.
References
- ASABE. (2012). ASAE S352. 2 moisture measurement–unground grain and seeds.
- Bechlin, T. R., Granella, S. J., Christ, D., Coelho, S. R. M., & Viecelli, C. A. (2019). Evaluation of grain and oil quality of packaged and ozonized flaxseed. Journal of Stored Products Research, 83, 311–316, 2019. 10.1016/j.jspr.2019.07.014
- Bernacchia, R., Preti, R., & Vinci, G. (2014). Chemical composition and health benefits of flaxseed. Austin Journal of Nutrition Food and Sciences, 2(8), 1045.
- Butz P, Tauscher B. Emerging technologies: Chemical aspects. Food Research International. 2002;35(2–3):279–284. doi: 10.1016/S0963-9969(01)00197-1. [DOI] [Google Scholar]
- Carbone K, Mencarelli F. Influence of short-term postharvest ozone treatments in nitrogen or air atmosphere on the metabolic response of white wine grapes. Food and Bioprocess Technology. 2015;8(8):1739–1749. doi: 10.1007/s11947-015-1515-y. [DOI] [Google Scholar]
- Clark, S., Ng, H., Wu, G., Jain, D., Bassi, G., Kandel, R., & Mazzulli, T. (2021). Comparative evaluation of four hydrogen peroxide-based systems to decontaminate N95 respirators. Antimicrobial Stewardship & Healthcare Epidemiology,1(1). 10.1017/ash.2021.183 [DOI] [PMC free article] [PubMed]
- Fan X, Song Y. Advanced oxidation process as a postharvest decontamination technology to improve microbial safety of fresh produce. Journal of Agricultural and Food Chemistry. 2020;68(46):12916–12926. doi: 10.1021/acs.jafc.0c01381. [DOI] [PubMed] [Google Scholar]
- Gayán, E., Condón, S., & Álvarez, I. (2014). Biological aspects in food preservation by ultraviolet light: A review. Food and Bioprocess Technology,7(1), 1–20. 10.1007/s11947-013-1168-7
- Glaze, W. H., Kang, J. W., & Chapin, D.H. (1987). The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone: Science & Engineering, 9(4), 335–352. 10.1080/01919518708552148
- Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M. Flax and flaxseed oil: An ancient medicine & modern functional food. Journal of Food Science and Technology. 2014;51(9):1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat, R. S., Singh, V. V., Sharma, P., & Rai, P. K. (2019). Oilseed brassica in India: Demand, supply, policy perspective and future potential. OCL, 26, 8. 10.1051/ocl/2019005
- Los A, Ziuzina D, Bourke P. Current and future technologies for microbiological decontamination of cereal grains. Journal of Food Science. 2018;83(6):1484–1493. doi: 10.1111/1750-3841.14181. [DOI] [PubMed] [Google Scholar]
- Mills, J., Sinha, R., & Wallace, H. (1978). Multivariate evaluation of isolation techniques for fungi. Techniques.
- Mohanan A, Nickerson MT, Ghosh S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chemistry. 2018;266:524–533. doi: 10.1016/j.foodchem.2018.05.117. [DOI] [PubMed] [Google Scholar]
- Mundhada, S., Chaudhry, M. M. A., Erkinbaev, C., & Paliwal, J. (2022). Development of safe storage guidelines for prairie-grown flaxseed. Journal of Stored Products Research, 97, 101965. 10.1016/j.jspr.2022.101965
- Nadimi, M., Divyanth, L. G., & Paliwal, J. (2022c). Automated detection of mechanical damage in flaxseeds using radiographic imaging and machine learning. Food and Bioprocess Technology. 10.1007/s11947-022-02939-5
- Nadimi, M., Loewen, G., & Paliwal, J. (2022b). Assessment of mechanical damage to flaxseeds using radiographic imaging and tomography. Smart Agricultural Technology, 2, 100057. 10.1016/j.atech.2022.100057
- Nadimi, M., Sun, D. W., & Paliwal, J. (2022a). Effect of laser biostimulation on germination of wheat. Applied Engineering in Agriculture, 38(1), 77–84. 10.13031/aea.14772
- O’Connor, T., & O’Brien, N. (2006). Lipid oxidation. In Advanced Dairy Chemistry, 2 lipids, pp. 557–600, Springer. 10.1007/0-387-28813-9_16
- Pandiselvam, R., Mayookha, V. P., Kothakota, A., Sharmila, L., Ramesh, S. V., Bharathi, C. P., Gomathy, K., & Srikanth, V. (2020). Impact of ozone treatment on seed germination–A systematic review. Ozone: Science & Engineering, 42(4), 331–346. 10.1080/01919512.2019.1673697
- Piechowiak T, Skóra B, Balawejder M. Ozone treatment induces changes in antioxidative defense system in blueberry fruit during storage. Food and Bioprocess Technology. 2020;13(7):1240–1245. doi: 10.1007/s11947-020-02450-9. [DOI] [Google Scholar]
- Piva GS, Weschenfelder TA, Franceschi E, Cansian RL, Paroul N, Steffens C. Linseed (Linum usitatissimum) oil extraction using different solvents. Food Technology and Biotechnology. 2018;56(3):366–372. doi: 10.17113/ftb.56.03.18.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raila, A., Lugauskas, A., Steponavicius, D., Railiene, M., Stepanoviciene, A., & Zvicevicius, E. (2006). Application of ozone for reduction of mycological infection in wheat grain. Annals of Agricultural and Environmental Medicine, 13(2). [PubMed]
- Saeidi G, Rowland GG. The effect of temperature, seed colour and linolenic acid concentration on germination and seed vigour in flax. Canadian Journal of Plant Science. 1999;79(3):315–319. doi: 10.4141/p98-100. [DOI] [Google Scholar]
- Savi, G. D., Piacentini, K. C., Bortolotto, T., & Scussel, V. M. (2016). Degradation of bifenthrin and pirimiphos-methyl residues in stored wheat grains (Triticum aestivum L.) by ozonation. Food Chemistry, 203, 246–251, 2016. 10.1016/j.foodchem.2016.02.069 [DOI] [PubMed]
- Schönemann A, Edwards HG. Raman and FTIR microspectroscopic study of the alteration of Chinese tung oil and related drying oils during ageing. Analytical and Bioanalytical Chemistry. 2011;400(4):1173–1180. doi: 10.1007/s00216-011-4855-0. [DOI] [PubMed] [Google Scholar]
- Shen, M. H., & Singh, R. K. (2022). Decomposing aflatoxins in peanuts using advanced oxidation processes by UV and H2O2. Food and Bioprocess Technology, 1–11. 10.1007/s11947-022-02844-x
- Sravanthi, B., Jayas, D. S., Alagusundaram, K., Chelladurai, V., & White, N. D. G. (2013). Effect of storage conditions on red lentils. Journal of Stored Products Research, 53, 48–53. 10.1016/j.jspr.2013.01.004
- Stitt, D. M., Kastyak-Ibrahim, M. Z., Liao, C. R., Morrison, J., Albensi, B. C., & Gough, K. M. (2012). Tissue acquisition and storage associated oxidation considerations for FTIR microspectroscopic imaging of polyunsaturated fatty acids. Vibrational Spectroscopy, 60, 16–22. 10.1016/j.vibspec.2011.10.016
- Van der Weerd J, Van Loon A, Boon JJ. FTIR studies of the effects of pigments on the aging of oil. Studies in Conservation. 2005;50(1):3–22. doi: 10.1179/sic.2005.50.1.3. [DOI] [Google Scholar]
- Vettraino AM, Vinciguerra V, Pacini G, Forniti R, Goffi V, Botondi R. Gaseous ozone as a suitable solution for postharvest chestnut storage: Evaluation of quality parameter trends. Food and Bioprocess Technology. 2020;13(1):187–193. doi: 10.1007/s11947-019-02378-9. [DOI] [Google Scholar]
- Vieira, S. A., Zhang, G., & Decker, E. A. (2017). Biological implications of lipid oxidation products. Journal of the American Oil Chemists’ Society, 94(3), 339–351. 10.1007/s11746-017-2958-2
- Wiercigroch, E., Szafraniec, E., Czamara, K., Pacia, M. Z., Majzner, K., Kochan, K., ... & Malek, K. (2017). Raman and infrared spectroscopy of carbohydrates: A review. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 185, 317–335. 10.1016/j.saa.2017.05.045 [DOI] [PubMed]
- You, F. M., Duguid, S. D., Lam, I., Cloutier, S., Rashid, K. Y., & Booker, H. M. (2016). Pedigrees and genetic base of flax cultivars registered in Canada. Canadian Journal of Plant Science, 96, no. 5, 837–852. 10.1139/cjps-2015-0337
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors can confirm that all relevant data are included in the article and/or its supplementary information files.