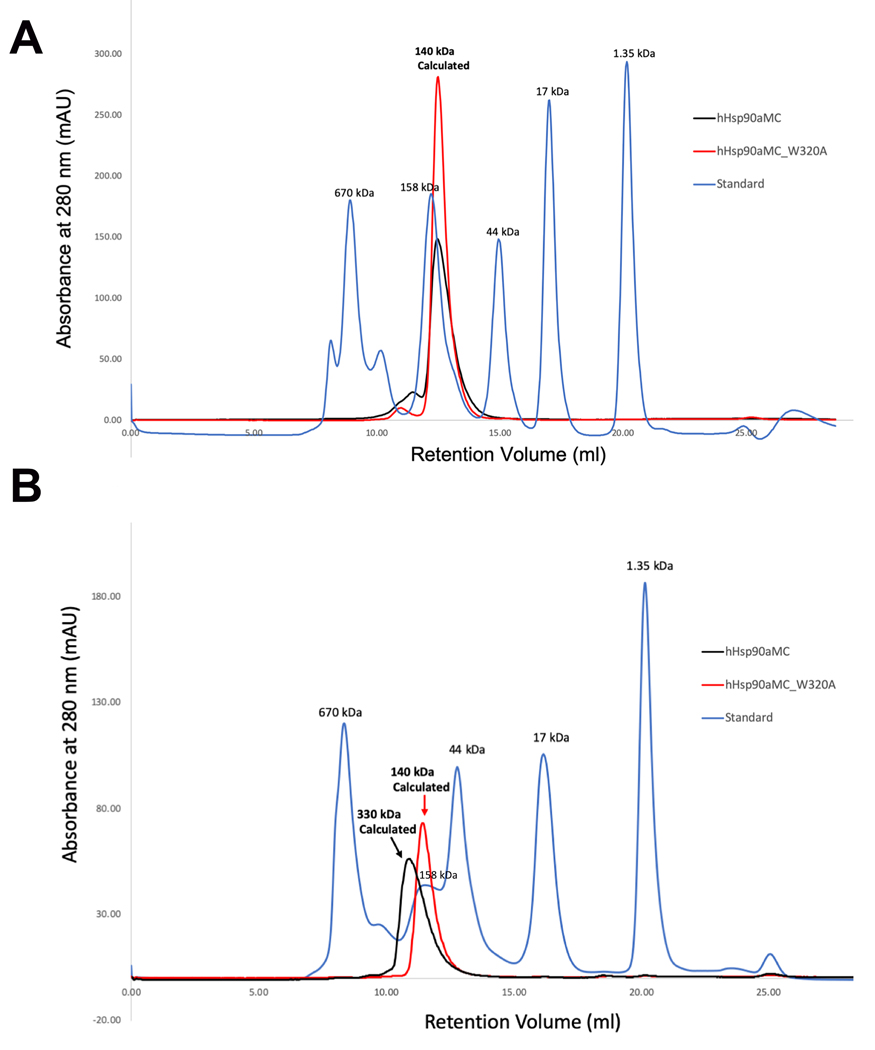
Figure 3.
Size-exclusion Chromatographs for WT Hsp90⍺MC and Hsp90αMC_W320A mutant proteins. (A). Chromatographs from fresh protein samples. The MWs are indicated for the protein peaks. The theoretic MW of hHsp90αMC is about 50kDa. Due to its elongated shape, the apparent MW is calculated as 67kDa in solution (www.fluidic.com) based on its hydrodynamic radius of 35 Å calculated from the current crystal structure(58). The estimated MW of the protein from the retention volume is about 140 kDa, representing a dimer in solution. (B).Chromatographs from the thawed protein samples after frozen at −80 oC. The retention volumes as well as the estimated MWs are indicated for the protein peaks. Note WT protein oligomerized while W320A mutant protein retained as a dimer in solution.