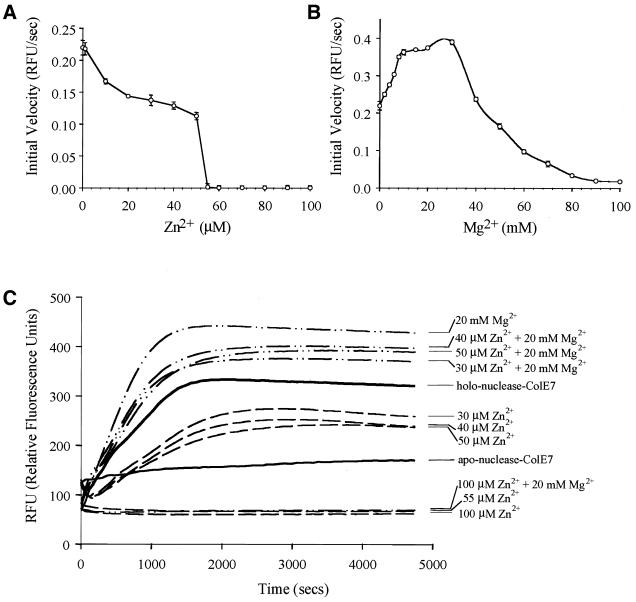
Figure 5.
Dependence of nuclease-ColE7 DNase activity on the concentrations of Zn2+ and Mg2+ measured by a fluorescent method using the fluorophore and quencher-labeled oligonucleotide as a substrate. (A) The initial velocity of DNA cleavage for the holo-nuclease-ColE7 was varied with Zn2+ ion concentration. The zinc ion inhibited the DNA cleavage and nuclease-ColE7 had no detectable DNase activity when Zn2+ concentrations (55 µM) were more than ∼1000-fold of enzyme concentration (60 nM). (B) The initial velocity of DNA cleavage for the zinc-containing holo-nuclease-ColE7 was varied with Mg2+ ion concentration. (C) The time course of fluorescence emission intensity caused by nuclease-ColE7 in buffers containing different divalent metal ions. The zinc-containing holo-nuclease-ColE7 had moderate activity; the apo-enzyme had no DNase activity; Zn2+ inhibited and Mg2+ (<40 mM) activated DNase activity of the holo-enzyme.