Summary
Background
Efficacy and safety of onasemnogene abeparvovec (OA) for Spinal Muscular Atrophy infants under 7 months and <8.5 kg has been reported in clinical trials. This study examines efficacy and safety predictors in a wide age (22 days–72 months) and weight (3.2–17 kg) range, also including patients previously treated with other drugs.
Methods
46 patients were treated for 12 months between January 2020 and March 2022. Safety profile was also available for another 21 patients with at least 6 month follow-up after OA infusion. 19/67 were treatment naïve when treated with OA. Motor function was measured with the CHOP-INTEND.
Findings
CHOP-INTEND changes varied among age groups. Baseline score and age at OA treatment best predicted changes. A mixed model post-hoc analysis showed that in patients treated before the age of 24 months the CHOP-INTEND changes were already significant 3 months after OA while in those treated after the age of 24 months the difference was only significant 12 months after OA. Adverse events occurred in 51/67. The risk for elevated transaminases serum levels was higher in older patients. This was also true for weight and for pre-treatment with nusinersen when analysed individually. A binomial negative regression analysis showed that only age at OA treatment had a significant effect on the risk of elevated transaminases.
Interpretation
Our paper describes OA 12-month follow-up showing efficacy across various age and weight groups not targeted by clinical trials. The study identifies prognostic factors for safety and efficacy in treatment selection.
Funding
None.
Keywords: Spinal muscular atrophy, Gene therapy, Follow-up, Longitudinal, Safety
Research in context.
Evidence before this study
In clinical trials gene therapy for SMA has been used in infants below 7 months of age and weight less than 8.5 kgs. Due to the recent approval, real world data using onasemnogene abeparvovec is now becoming increasingly available. We conducted a systematic literature search with no language restrictions on PubMed, from database inception to December 20, 2022 with the search terms (((Spinal Muscular Atrophy [MeSH Terms]) AND (onasemnogene abeparvovec)) OR (zolgensma)) NOT (review [Publication Type]). Twenty-three studies were taken into consideration. The recent real world papers expand efficacy and safety data to heavier and older infants but with a short-term follow-up. Data on efficacy in older infants following switch from other therapies is also limited.
Added value of this study
This paper represents the first systematic description of 12 month data in a large cohort of 67 children with spinal muscular atrophy, exploring possible prognostic factors for both safety and efficacy in different age and weight groups. Additionally, it pays particular attention to the children who received gene therapy after previous treatment with another disease modifying therapy.
Implications of all the available evidence
The data on 12 month follow-up of onasemnogene abeparvovec in a cohort of children with weight and age wider than those reported in clinical trials provides the opportunity to set up the right expectations of the efficacy of gene therapy if administered at different ages. The additional data on efficacy following switch from a previous disease modifying therapy provides information will be of help for families and clinicians at the time they face the choice if and when to switch to the new therapy.
Introduction
Spinal muscular atrophy (SMA) is caused by biallelic mutations (deletions or small mutations) of the survival motor neuron gene 1.1 Before the advent of new therapies by the age of 2 years over 90% of infants with SMA type I, the most severe form, did not survive or needed permanent ventilation.2,3 Disease modifying therapies and supportive care have significantly changed the course of the disease.4 Onasemnogene abeparvovec (OA) is an adeno-associated viral (AAV9) vector-based gene replacement therapy. Recent clinical trials show a dramatic increase in survival and function.5, 6, 7, 8 These findings have been confirmed in other studies reporting long term follow-up of the initial trial9 and by real-world data.10, 11, 12 The clinical trials only included infants below the age of 7 months and 8.5 kg,5, 6, 7 but the drug has subsequently been approved for a wider range of patients.
Following the availability of OA, many families whose children had been treated soon after diagnosis with nusinersen that until then had been the first drug available, have opted for switching to the new available therapy. The decision was often taken in order to avoid repeated intrathecal injections or was driven by the exciting results obtained in younger infants.10, 11, 12
Recent real-world data in a large cohort suggest that the use of OA in older patients is associated with a more limited improvement than that observed in infants treated before the age of 6 months10, 11, 12 and that previous exposure to nusinersen and older age may increase the risk for elevated transaminases.12
We report our experience in 67 patients treated with OA trying to establish possible prognostic factors for safety and efficacy in patients treated at different ages, paying special attention to those previously treated with other drugs.
Methods
The cohort includes 67 patients with homozygous deletions of SMN1 treated with OA between January 2020 and March 2022 who had at least 6 month follow-up, with 46/67 reaching 12 month follow-up. Only type I infants could be treated, in line with the Italian regulatory label. As part of the activities of an Italian nation-wide registry, data were prospectively collected using a structured electronic case report form (eCRF).13 The study was approved by the institutional Ethics Committee (2355/18 ID:1894) and all parents signed consent forms.
Nineteen patients were treatment naïve at the time they were treated with OA. Another two had been previously treated with risdiplam and 46 with nusinersen. The number of doses of nusinersen ranged between 1 and 15 (mean number of doses: 5.24) and, in all patients, OA was administered approximately 3 months after the last nusinersen dose. All patients received the standard dose of 1.1 × 1014 vg/kg OA intravenously over an hour. Oral prednisolone treatment was started on the day before administration of OA at a standard dose of 1 mg/kg/day and continued for at least 4 weeks. Steroid dose was adapted according to clinical needs. Blood tests, including full blood count and liver enzymes were performed and they were always found to be normal.
Follow-up visits including lab tests were scheduled on day 2, 7, and then every week for at least 4 weeks, followed by biweekly intervals for 2 months, and monthly intervals for another 3 months. Additional visits were scheduled when needed on the basis of clinical or laboratory abnormalities.
Functional assessments
All patients were assessed using the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND), which includes 16 items with a total score between 0 and 64 (higher score reflects a higher level of motor function). Details of the training and reliability sessions have already been reported.14 Patients were assessed at the time treatment started (T0), after 3 months (T3), after 6 months (T6), and at 12 months (T12) from the infusion.
Sitting posture
In the registry sitting posture is defined as the ability to sit unsupported and without brace for at least 3 s (item 1 of the Hammersmith Functional motor scale).
Laboratory tests and investigations
Complete blood count, kidney and liver function, creatine kinase, and troponin levels were measured at the following time-points: day 2, 7, and then every week for at least 4 weeks, followed by biweekly intervals for 2 months, and monthly intervals for another 3 months. In the event of abnormal liver enzymes in the first weeks determination of Aspartate Transferase (AST) and Alanine Transaminase (ALT) were repeated every three days until normalization. Cardiac evaluation with echocardiography and electrocardiogram were also performed at baseline and at 12 months or in the presence of elevated troponin levels.
Statistical analysis
The cohort was stratified according to sex, SMN2 copy number, age at treatment initiation, weight at treatment initiation (≤8.5 kg, >8.5 kg). The cutoff point of 8.5 kg was selected as this was used in the pivotal clinical trials. Disease severity was defined using the Dubowitz decimal classification,15 in 1.1 (most severe patients), 1.5 (intermediate), and 1.9 (mildest phenotypes).
Simple descriptive statistics were used. The median and interquartile range value (IQR), mean and standard deviation (SD) were evaluated.
Paired samples Wilcoxon test was used to assess differences between T12 and T0 for the entire cohort and by stratification groups. Kruskal–Wallis test was conducted to examine the differences on CHOP-INTEND changes at 12 months post OA infusion according subgroups of stratification.
A linear mixed model with patient as random effect was fitted to predict CHOP-INTEND score with the possible confounder of stratification. T-tests were computed using Satterthwaite’s method. The analysis was performed using the R package lme4 and lmerTest.
To assess the effect of the different stratification variables on time-to-sit acquisition, the hazard ratio and its 95% confidence interval were calculated with the Cox regression model (univariable and multivariable).
Safety assessments
AST and ALT serum levels were defined as elevated when more than twice the upper limit of normal.16 Mean value and 95% confidence interval/standard deviation were reported as values for the different segments of the population. Kruskal–Wallis-test/Mann–Whitney test was use to assess the association between AST and ALT maximum elevation and age, weight, SMA type, SMN2 copy number, and sex. Negative binomial regression adjusted per days of follow-up (offset term) was used to examine whether the number of abnormal ALT and AST peaks were increased or decreased depending on sex, age at treatment initiation, weight at treatment initiation, and pre-treatment with nusinersen.
Role of the funding source
This research received no external funding. Data are available on request.
Results
Patients
At the time of OA treatment, 66/67 (98.5%) patients had a weight between 3.2 and 13.5 kg. Their age was between 22 days and 58 months. The remaining child, with a weight of 17 kg, was treated off label at the age of 72 months.
Four of the 67 (5.97%) were identified through neonatal screening and were asymptomatic at the time they were treated. The remaining 63 (94.03%) were classified according to the Dubowitz’s decimal classification: nine as 1.1, 45 as 1.5 and nine as 1.915 (Table 1).
Table 1.
Details of the whole cohort, of the naïve patients, and of those previously treated.
| Whole cohort (N = 67) | Naive (N = 19) | Pre-treated (N = 48) | 12-month cohort (N = 46) | |
|---|---|---|---|---|
| Age (months) | ||||
| Mean (SD) | 20 (17) | 3 (2.49) | 26 (17.49) | 22 (18.26) |
| Median (range) | 12 (0–72) | 3 (0–10) | 25 (3–72) | 18 (1–72) |
| Weight (kg) | ||||
| Mean (SD) | 8.29 (2.69) | 5.82 (2.49) | 9.23 (2.41) | 8.85 (2.65) |
| Median (range) | 8.1 (3.2–17.0) | 5.6 (3.2–9.2) | 9 (5.4–17.0) | 9 (4.1–17) |
| Male | 44 | 11 | 34 | 32 |
| Female | 23 | 10 | 13 | 14 |
| SMA type (N) | ||||
| Pre-symptomatic | 4 | 2 | 4 | 2 |
| SMA I | 62 | 16 | 62 | 44 |
| SMN2 copies (N) | ||||
| 2 | 62 | 17 | 62 | 42 |
| 3 | 4 | 1 | 4 | 4 |
| CHOP-INTEND patients' distribution per time-points | ||||
| T0 | 67 | 19 | 48 | 46 |
| T3 | 65 | 19 | 46 | 46 |
| T6 | 65 | 19 | 46 | 46 |
| T12 | 46 | 9 | 39 | 46 |
Efficacy
CHOP-INTEND
Details of the CHOP-INTEND scores over time in patients subdivided according to age at treatment and treatment status are available in Supplementary Figure S1.
Forty-six of 67 (68.66%) patients had at least a CHOP-INTEND assessment at the time treatment started (T0) and at 12 months (T12) from the infusion (range 11.5–12.9 months).
A paired Wilcoxon test, conducted to evaluate whether patients showed greater CHOP-INTEND scores with time, showed a significant difference (P < 0.0001) (Table 2). The Kruskal–Wallis test was used to examine the differences on CHOP-INTEND changes at 12 months post OA infusion according to subgroups of stratification (Table 2).
Table 2.
12-month CHOP-INTEND results on the sample of 46 patients of Wilcoxon test and Kruskal–Wallis-test/Mann–Whitney test according to the subgroups of stratification.
| Stratification subgroups | Time | Total (N) | Median (IQR) | Mean (SD) | P-value |
Median change |
Mean change |
P-value |
|---|---|---|---|---|---|---|---|---|
| Wilcoxon | T12–T0 (IQR) | T12–T0 (SD) | Kruskal–Wallis | |||||
| Age at OA treatment | ||||||||
| <6 months | T0 | 15 | 30 (20) | 34.4 (14.5) | P = 0.001b | 17 (12) | 16.7 (7.56) | Chi square = 15.13. P = 0.0005b, df = 2 |
| T12 | 15 | 51 (19) | 51.1 (10.8) | |||||
| 7–24 months | T0 | 12 | 42.5 (5.75) | 42.1 (8.27) | P = 0.003b | 6 (12) | 9.75 (8.4) | |
| T12 | 12 | 52.5 (9.5) | 51.8 (7.07) | |||||
| >24 months | T0 | 19 | 50 (8.5) | 47.1 (10.3) | P < 0.001b | 3 (6.5) | 5.11 (5.20) | |
| T12 | 19 | 53 (8.0) | 52.2 (9.15) | |||||
| Sex | ||||||||
| Female | T0 | 14 | 43.5 (22.8) | 41.2 (14.5) | P = 0.001b | 8.5 (813.8) | 10.8 (9.23) | Chi square = 0.16,529. P = 0.68, df = 1 |
| T12 | 14 | 53 (8.5) | 52 (7.81) | |||||
| Male | T0 | 32 | 43.5 (13.2) | 41.8 (11.7) | P < 0.001b | 8.5 (14) | 9.78 (8.16) | |
| T12 | 32 | 52 (11.5) | 51.6 (9.69) | |||||
| Weight | ||||||||
| <8500 gr | T0 | 20 | 36.5 (20) | 36.7 (13.4) | P = 0.001b | 17 (13.2) | 15 (8.48) | Chi square = 11.31 P < 0.001b, df = 1 |
| T12 | 20 | 51.5 (14.2) | 51.8 (9.64) | |||||
| >8500 gr | T0 | 26 | 47 (10.8) | 45.5 (10.4) | P < 0.001b | 4 (6.75) | 6.27 (6.16) | |
| T12 | 26 | 53.5 (10.2) | 51.7 (8.81) | |||||
| SMN2 copy number | ||||||||
| 2 | T0 | 42 | 42.5 (19) | 40.4 (12.0) | P < 0.001b | 8.5 (14.8) | 10.3 (8.44) | N/A |
| T12 | 42 | 52.0 (13.2) | 50.8 (8.83) | |||||
| 3 | T0 | 4 | 55.5 (18) | 54.5 (11.1) | N/A | 6.5 (14) | 7.5 (8.81) | |
| T12 | 4 | 64 (2.0) | 62.0 (4.0) | |||||
| SMA 1 severity of the disease | ||||||||
| 1.1 | T0 | 4 | 30 (13) | 29.5 (8.19) | N/A | 14 (9.5) | 15.5 (6.81) | Chi square = 6.31 P = 0.04b, df = 2 |
| T12 | 4 | 44.5 (11) | 45 (12.0) | |||||
| 1.5 | T0 | 32 | 43 (19) | 39.8 (11.7) | P < 0.001b | 10.5 (15.2) | 11.3 (8.56) | |
| T12 | 32 | 52 (10.2) | 51.1 (8.31) | |||||
| 1.9 | T0 | 8 | 51 (8) | 50 (8.18) | P = 0.035b | 3 (4.5) | 4.62 (5.78) | |
| T12 | 8 | 54 (14) | 54.6 (9.02) | |||||
| Pre-treatment with nusinersena | ||||||||
| No | T0 | 7 | 30 (9.5) | 30.4 (9.03) | P = 0.022b | 17 (9.5) | 16.6 (6.32) | Chi square = 0.030, P = 0.8617, df = 1 |
| T12 | 7 | 47 (9.5) | 47 (10.3) | |||||
| Yes | T0 | 8 | 38.5 (25.8) | 37.9 (18.0) | P = 0.022b | 19.5 (11.2) | 16.8 (8.94) | |
| T12 | 8 | 59 (14.0) | 54.6 (10.6) |
Computed only in patients <6 months at OA treatment.
Reached statistical significance (P < 0.05).
Mixed model analysis
A linear mixed model was used to analyse the effects of the stratification variables on 12-month results on CHOP-INTEND scores considering random variation across participants. The interaction of age at treatment with time, and the CHOP-INTEND score at baseline resulted to have a significant effect (P < 0.001) on CHOP-INTEND scores at 12 months. The post-hoc analysis conducted on the interaction between time and age at treatment showed a significant increase of the CHOP-INTEND at different time-points depending on the age at treatment (Table 3).
Table 3.
Model parameter estimates for the mixed-effect model with CHOP-INTEND score as dependent variables.
| Parameter | CHOP |
||
|---|---|---|---|
| Estimate (95% CI) | SE | P-value | |
| Intercept | 6.27 (−0.08, 12.61) | 3.21 | 0.053 |
| Time | |||
| T0 (reference level) | |||
| T3 | 11.33 (8.85, 13.81) | 1.26 | <0.001a |
| T6 | 13.93 (11.45, 16.41) | 1.26 | <0.001a |
| T12 | 16.67 (14.19, 19.15) | 1.26 | <0.001a |
| Sex | |||
| Female (reference level) | |||
| Male | −1.00 (−3.89, 1.90) | 1.46 | 0.500 |
| Age | |||
| <6 months (reference level) | |||
| 7–24 months | 1.32 (−3.92, 6.57) | 2.65 | 0.499 |
| >24 months | 2.65 (−3.76, 9.07) | 3.25 | 0.81 |
| Pre-treatment with nusinersen | |||
| No (reference level) | |||
| Yes | 1.39 (−2.71, 5.49) | 2.07 | 0.506 |
| Weight at treatment | |||
| <8500 gr (reference level) | |||
| >8500 gr | −0.72 (−5.06, 3.62) | 2.20 | 0.746 |
| Disease severity | |||
| 1.1 (reference level) | |||
| 1.5 | 2.12 (−2.88, 7.11) | 2.53 | 0.408 |
| 1.9 | 0.29 (−5.47, 6.05) | 2.92 | 0.921 |
| CHOP score at baseline | 0.77 (0.64, 0.91) | 0.07 | <0.0001a |
| SMN2 copy number | |||
| 2 (reference level) | |||
| 3 | 2.13 (−2.97, 7.23) | 2.58 | 0.415 |
| Time∗Age at treatment | |||
| T3∗7–24 months | −5.58 (−9.30, −1.86) | 1.88 | 0.0036a |
| T6∗7–24 months | −6.43 (−10.15, −2.71) | 1.88 | <0.0001a |
| T12∗7–24 months | −6.92 (−10.64, −3.20) | 1.88 | <0.0001a |
| T3∗>24 months | −8.70 (−12.02, −5.38) | 1.68 | <0.0001a |
| T6∗>24 months | −10.09 (−13.41, −6.77) | 1.68 | <0.0001a |
| T12∗ > 24 months | −11.56 (−14.88, −8.24) | 1.68 | <0.0001a |
| Scaled residuals | ||||
|---|---|---|---|---|
| Min | 1Q | Median | 3Q | Max |
| −2.43 | −0.56 | 0.04 | −0.56 | 2.86 |
| Random effects | |||
|---|---|---|---|
| Groups | Name | Variance | Std. Dev. |
| id2 | (Intercept) | 12.50 | 3.54 |
| Residual | 11.84 | 3.44 | |
| Number of obs: 184, groups: id2, 46 | |||
Reference level used for comparison are labelled in the table. Only the 46 patients who had completed 12 months follow-up were analysed. Supplementary Table S1 provides post-hoc contrast for time∗age at treatment, that should be interpreted with caution seen the relative sample size used.
Significant values (α < 0.05).
Stratification of CHOP-INTEND 12-month changes
Fig. 1 shows details of the patients who reached cut off points of 40, 50, and 60 on the CHOP-INTEND in the naïve and previously treated cohorts at baseline and after 12 months.
Fig. 1.
Details of the symptomatic children who reached cut off points of 40, 50, and 60 on the CHOP-INTEND in the naïve and previously treated cohorts at baseline and after 12 months.
Achievement of sitting posture
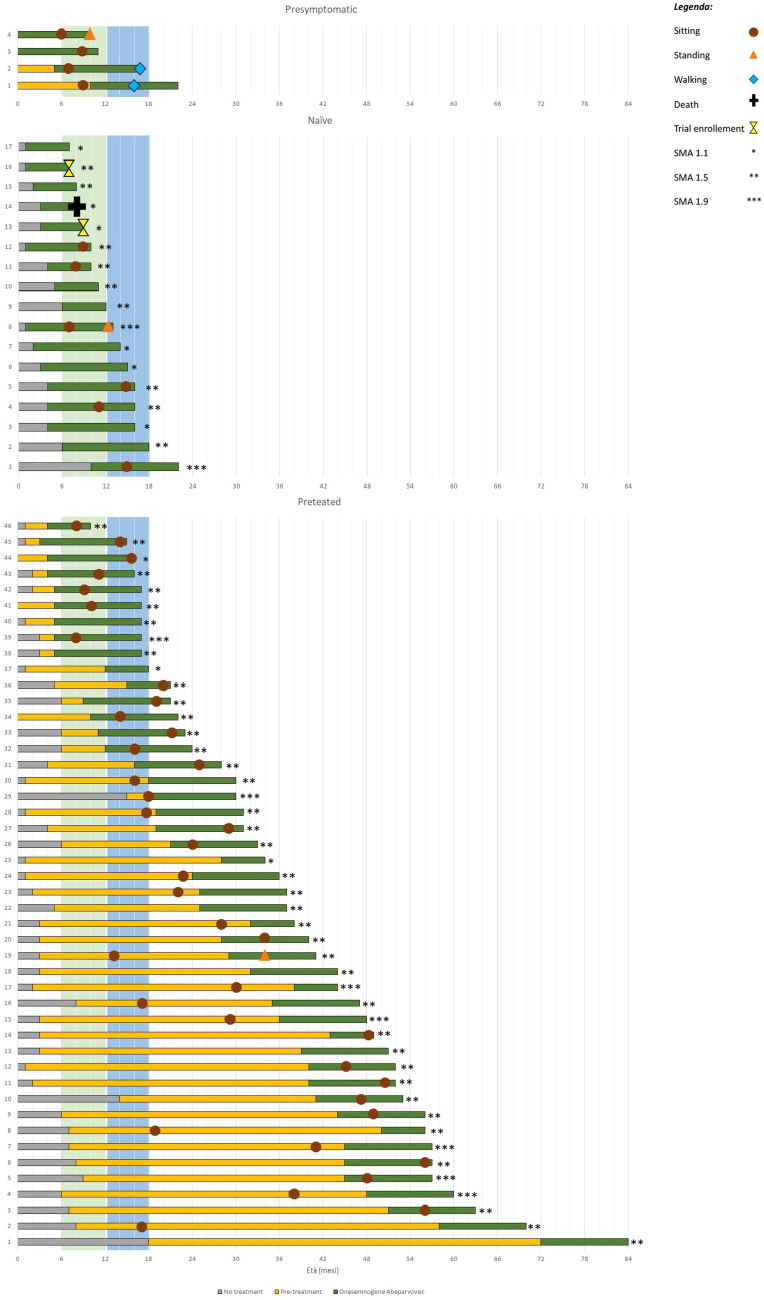
Sitting was achieved in all 4 pre-symptomatic patients (100%, mean age 7.75 months; mean age at last follow-up 15 months). One acquired independent standing at 10 months (25%) and the two older patients also acquired independent walking (50%, at 16 and 17 months, respectively).
In the 16 surviving naïve symptomatic patients (100%) sitting was achieved in 6 (37.5%) (mean age at sitting: 11 months; mean age at last follow-up 13 months). One (6.25%) also acquired independent standing at 12 months.
In the 46 pre-treated symptomatic patients (100%) sitting was achieved in 38/46 (83%) (mean age at sitting 27 months; mean age at last follow-up 37 months). Fourteen of the 38 (37%) had achieved independent sitting before OA. Of the remaining 24 (63%) who achieved sitting after OA started 12 (50%) only had the loading dose of nusinersen, generally in the first months after birth. One patient also acquired independent standing at 34 months. The remaining 8/46 (17.4%) did not achieve sitting even after switching to OA (Fig. 2).
Fig. 2.
Individual results on motor milestone achievement. The green lines indicate treatment with onasemnogene abeparvovec, the orange lines treatment with previous treatment, and the grey ones indicate the time without any treatment.
Table 4 reports the results of univariable and multivariable Cox regression model analyses. Patients who had acquired sitting before OA administration were not included in the regression (n = 15). SMA severity, duration of previous treatment, and SMN2 copies were the only statistically significant prognostic factors upon univariable analysis. SMA severity and duration of previous treatment retained significance upon multivariable analysis.
Table 4.
Hazard ratios for all prognostic factor of sitting acquisition in patients treated with OA.
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Sma severity | ||||||
| 1.1 (ref) | ||||||
| 1.5 | 3.50 | 0.82–14.97 | 0.090 | 1.48 | 0.29–7.35 | 0.631 |
| 1.9 | 17.92 | 3.11–103.17 | 0.001a | 25.87 | 2.87–232.45 | 0.003a |
| Pre-symptomatic | 10.84 | 1.75–67.06 | 0.010a | 28.26 | 2.71–294.18 | 0.005a |
| Age at OA infusion | ||||||
| <6 months (ref) | ||||||
| 7–24 months | 1.83 | 0.77–4.30 | 0.169 | 1.29 | 0.20–8.47 | 0.788 |
| >24 months | 1.25 | 0.53–2.94 | 0.605 | 0.51 | 0.04–6.90 | 0.615 |
| Duration of previous treatment (naïve <6 months> 6 months) | ||||||
| Naïve (ref) | ||||||
| <6 months | 2.78 | 1.01–7.70 | 0.048a | 8.59 | 1.90–38.82 | 0.005a |
| >6 months | 2.68 | 1.03–6.93 | 0.042a | 22.44 | 3.01–167.19 | 0.002a |
| SMN2 copy number | ||||||
| 2 SMN2 copies (ref) | ||||||
| 3 SMN2 copies | 3.47 | 1.18–10.13 | 0.022a | 2.14 | 0.42–10.80 | 0.358 |
| Weight at OA infusion | ||||||
| <8.5 kg (ref) | ||||||
| ≥8.5 kg | 1.36 | 0.67–2.78 | 0.389 | 0.73 | 0.18–2.86 | 0.656 |
| Sex | ||||||
| Male (ref) | ||||||
| Female | 1.26 | 0.61–2.65 | 0.526 | 1.48 | 0.47–4.68 | 0.507 |
OA, onasemnogene abeparvovec; SMN2 copy number, survival motor neuron 2 copy number.
Significant values (α < 0.05).
Of the 52 included in the regression analysis, 32 acquired sitting during the OA follow-up. The median time of sitting acquisition was 16.5 (IQR 27) months (Supplementary Table S2, Supplementary Figure S2).
Bulbar and respiratory function
Thirty-six of the 46 children with one-year follow-up (78%) did not need for nutritional support at baseline; after 12 months from the OA, they all remained orally fed. The remaining ten (22%) remained tube feeding.
Ten patients (22%) did not need non-invasive ventilation at baseline; nine of them (90%) remained on spontaneous breathing after 12 months, only one needed for non-invasive ventilation <10 h/day (SMA I 1.5). Of the remaining 36 requiring non-invasive ventilation (76%), 32 needed NIV for <10 h/day, and four for >10 h/day at baseline. After 12 months from OA, one showed a reduction in the duration of daily ventilation to <10 h/day and another one stopped ventilation completely.
Safety
Within the first 7 days after treatment, 15 (22.4%) patients had pyrexia, and 14 (20.9%) vomiting or loss of appetite. Of 67 patients, 26 (39%) showed at least one episode of 2-fold AST increase. Of these 26, 17 were also showing 2-fold ALT increase. In total, on the 67 patients included in the study, 20 showed at least one episode of 2-fold ALT increase (Fig. 3 and Supplementary Figure S3). Supplementary Table S3 shows details on patient characteristics and median value of the increase.
Fig. 3.
Individual trajectories of x-fold AST and ALT subdivided by age at treatment. Panel A: x-fold AST (aspartate aminotransferase); Panel B: x-fold ALT (alanine aminotransferase).
Table 5 reports descriptive and inferential statistics on the association between the maximum elevation of AST and ALT and patient’s baseline characteristic.
Table 5.
Descriptive and inferential statistics on the association between the maximum elevation of AST and ALT and patient’s baseline characteristic.
| AST (x-fold) |
ALT (x-fold) |
|||||
|---|---|---|---|---|---|---|
| Median (range) | IQR | P-value | Median (range) | IQR | P-value | |
| Sex | ||||||
| Female (n = 22) | 0.83 (−0.11–11.8) | 2.05 | U | 1.39 (−0.56–13.0) | 3.17 | U |
| Male (n = 45) | 0.67 (−0.58–12.5) | 2.42 | P = 0.7997 | 0.72 (−0.68–13.9) | 2.44 | P = 0.6937 |
| Type | ||||||
| Pre-symptomatic (n = 4) | 0.36 (−0.11–11.8) | 3.43 | H (3) = 1.628 | −0.01 (−0.34–13.0) | 3.51 | H (3) = 3.9225 |
| 1.1 (n = 9) | 0.53 (−0.05–3.78) | 0.89 | P = 0.653 | −0.08 (−0.16–5.20) | 0.76 | P = 0.27 |
| 1.5 (n = 45) | 0.89 (−0.58–12.5) | 2.64 | 0.80 (−0.68–13.9) | 3.24 | ||
| 1.9 (n = 9) | 1.04 (0.29–2.53) | 1.62 | 1.48 (−0.02–8.62) | 1.14 | ||
| SMN2 copies | ||||||
| 2 (N = 63) | 0.71 (−0.58–12.5) | 2.32 | N/A | 0.72 (−0.68–13.9) | 2.77 | N/A |
| 3 (n = 4) | 1.60 (0.34–2.93) | 2.04 | 0.88 (0.10–3.3) | 0.92 | ||
| Age at treatment | ||||||
| <6 (n = 29) | 0.38 (−0.58–5.15) | 0.71 | H (2) = 10.7 | 0.10 (−0.68–9.14) | 1.12 | H (2) = 9.6569 |
| 7–24 (n = 14) | 2.44 (0.38–11.8) | 2.92 | P = 0.005a | 1.14 (−0.32–13.0) | 4.82 | P = 0.008a |
| >24 (n = 24) | 1.90 (−0.22–12.5) | 3.49 | 2.05 (−0.58–13.9) | 2.75 | ||
| Weight at treatment | ||||||
| ≤8.5 kg (n = 35) | 0.45 (−0.58–5.38) | 0.74 | U | 0.12 (−0.68–9.14) | 1.09 | U |
| >8.5 kg (n = 32) | 2.31 (−1.13–12.5) | 3.25 | P = 0.002a | 2.65 (−0.58–13.9) | 4.01 | P = 0.0001a |
| Pre-treatment with nusinersen | ||||||
| No (n = 20) | 0.34 (−0.58–2.27) | 0.51 | U | −0.03 (−0.68–8.62) | 1.22 | U |
| Yes (n = 47) | 1.56 (−0.22–12.5) | 3.36 | P = 0.0007a | 1.42 (−0.58–13.9) | 3.37 | P = 0.010a |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; U, Mann–Whitney U test; H, Kruskal–Wallis H test; IQR, interquartile range.
Significant values (α < 0.05).
None of the patients had any clinical sign of acute liver failure or increase in bilirubin. The increased AST and ALT (more than twice normal values) detected in the first weeks were always treated adjusting prednisolone from 1 to 2 mg/kg per day and there was never the need to further increase the dose of steroids or use additional drugs. For the peaks occurring at the end of the first month or after, these were generally treated by deciding to maintain the same steroid dose and delay tapering if still on steroids or, in those who had already completed the tapering, by reintroducing 1 mg/kg.
Conducting a negative binomial regression analysis adjusting per days of follow-up, the risk of having abnormal AST results in patients with an age at treatment >24 months was 5.76 times elevated if compared to patients <6 months, and in patients with an age at treatment between 7 and 24 months was 7.52 times elevated if compared to patients <6 months. The risk of having abnormal ALT results in patients with an age at treatment >24 months was 11.08 times elevated if compared to patients <6 months, and in patients with an age at treatment between 7 and 24 months was 9.07 times elevated if compared to patients <6 months (Supplementary Table S4).
Thrombocytopenia (<150 × 109/L) occurred in 35 (52%) of 67 patients at a mean of 5–7 days after treatment. Six of the 35 (17.14%) had a thrombocyte count below 50 × 109/L and were treated adjusting the prednisone dose. None developed petechiae, bleeding, or required blood transfusion. Thrombotic microangiopathy was not observed.
Of 35 patients with thrombocytopenia, four were treatment naïve (11%) while 31 were previously treated (89%). Thrombocytopenia was more frequent in older and heavier patients and in those previously treated but this did not reach statistical significance.
Transient mild increase in troponin were found in 3/67 in the second week. Clinical examination, ECG, and echo were normal in all. None of the patients had obvious signs of sensory root ganglia dysfunction on a standard neurological examination. We did not observe any treatment related serious adverse events. One child showed hemophagocytic lymphohistiocytosis. One child developed hydrocephalus 1 month after dosing.
One patient died at 8 months of age for traumatic event not related to OA treatment and another died of acute encephalitis occurring after 13 months from dosing.
Twenty-three (34.3%) had admissions related to COVID or other respiratory infections during the follow-up period.
Discussion
Our paper expands the available data on OA also exploring possible prognostic factors for safety and efficacy, paying particular attention to the children who were at a larger weight than those enrolled in the clinical trials and those who received OA after previous treatment with another disease modifying therapy. This topic is of particular interest at the time OA is becoming increasingly available in several countries where other therapies were already available and families and clinicians face therapeutic choice. Until recently, the choice could only be based on the results available from the clinical trials that however were targeting naïve young infants below 7 months6,7,9 or pre-symptomatic infants.17,18 The recently published real-world data mainly report short term data in cohorts with wider age and weight range than those used in clinical trials, also including infants previously treated with other disease modifying therapies.10, 11, 12,19, 20, 21, 22, 23, 24, 25
In our large cohort, also including patients older than 7 months and heavier than 8.5 kg, there was a significant CHOP-INTEND improvement between T0 and T12. Not surprisingly the best efficacy was observed in the few patients identified through neonatal screening and in the younger ones. Patients ≤6 months or ≤8.5 kg improved more than those >6 months or >8.5 kg. Since around 80% of the patients with an age >6 months at OA treatment had also a weight >8.5 kg, this information should be read as complementary.
In our cohort the mixed model post-hoc analysis showed that while in patients treated before the age of 24 months the CHOP-INTEND changes were already significant after 3 months and remained significant also at 6 and 12 months, in those treated after the age of 24 months the difference was only significant between T0–T12. The discrepancy with previous findings reporting no significant changes in infants treated after the age of 24 months12 can be explained by the fact that their follow-up was limited to 6 months while the significance in our cohort was only reached at 12 months.
Special attention was paid to the patients previously treated with other disease modifying therapies. With few exceptions, our patients had been treated with nusinersen but the possibility to perform a meaningful analysis of treated vs untreated in the individual age groups was limited by the fact that the great majority of the children >6 months had previously been treated while most younger patients were treatment naïve or only few had a short exposure to nusinersen until OA became available.
A linear mixed model considering random variation across participants and adjusting for different variables showed that the CHOP-INTEND score at baseline and age at OA treatment were the best predictors of the CHOP-INTEND changes at the different time-points while previous treatment and the other variables were not. These findings are in agreement with other studies also reporting age and baseline as predictors of outcome in smaller cohorts of older and heavier children.10,11,24,26
One of the main questions when switching to OA in patients who had already achieved some improvement as a response to the first therapy is whether the new drug will provide additional achievements. When we analysed if and when sitting had been achieved in our cohort we found multiple patterns. Approximately a third of the symptomatic patients had already achieved sitting at the time OA was administered. Others achieved sitting after switching to OA but the switch occurred before the age of 6 months, when sitting is not yet expected. More than a third achieved sitting after switching to OA and after a variable exposure to nusinersen. The remaining six never achieved sitting irrespective of the nusinersen or OA duration of treatment. The interpretation of these findings is difficult because of the heterogeneity of age, duration of previous treatment, and several other variables. The univariable Cox regression analyses assessing the effect of the different variables on the time-to-sit acquisition showed that decreasing SMA severity, increasing duration of previous treatment, and increasing SMN2 copies were the only statistically significant prognostic factors.
The safety profile in our cohort appeared to be overall similar to that reported in clinical trials. Survival was approximately 97%, in line with the clinical trials findings in younger infants. In the two children who did not survive the cause of death was not related to treatment. We did not observe any of the major serious adverse effects recently reported such as TMA10,19,27,28 or liver failure,19,20 irrespective of age or previous treatment.
Our paper also provides additional information on safety data and on the possible difference in adverse events in patients who were previously treated. Decreased platelets and elevated liver enzyme were the most frequent findings. The peaks of transaminases were never associated with clinical signs of liver failure with no need to increase the steroid dose beyond 2 mg/kg per day or use additional drugs.
As reported in clinical trials, AST, and ALT elevations were frequent in the first 14 days after dosing or between 4 and 6 weeks, as part of the previously reported bimodal time course,11,12,20,29 but we also observed later onset peaks occurring after the first month up to 13–14 weeks. Some of them were isolated peaks and not the previously reported persistent high value spanning over the first months20,29,30 and were not always preceded by a peak in the first weeks. These findings highlight the need for long term monitoring even in the patients who did not present the more commonly observed first peaks within 14 days.
The overall risk of increased liver enzyme was higher in older patients who, at variance with patients treated below the age of 6 months, had a higher risk of also developing elevations after the first weeks, as also reported by previous studies.12,29 This was confirmed by the x-fold analysis showing how AST and ALT were differently elevated among patients with different age at OA treatment, with younger patients having a median level of x-fold elevation lower that the older patients (0.38 (−0.58–5.15) vs. 02.44 (0.38–11.8) vs. 1.90 (−0.22–12.5)). This was also true for weight at the time of treatment and for pre-treatment with nusinersen when analysed individually. As all variables are obviously strictly correlated with one another, we further explored the possible effect of the individual factors. The binomial negative regression analysis adjusted per time of follow-up showed that only age at OA treatment had a significant effect on having one or more 2-fold elevations while weight and previous treatment were not. These findings are at variance with what reported by a recent large cohort study suggesting that previous treatment with nusinersen was per se a prognostic risk for developing increase in ALT and AST.12 The differences may be explained by the different cohorts studies as the previous study also included a number of naïve type II SMA patients treated after the age of 6 months who were not present in our cohort. This highlights one of the limitations of the study, i.e. the exclusion of type II and III and the small number of infants with 3 SMN2 copies.
In conclusion, our results confirm the efficacy of OA in children treated at different ages. We also provided further insights on safety and on the need to prolong the monitoring of liver enzymes beyond the first months. Our data also provide additional evidence of the response to OA when administered in older infants who have already been treated with another treatment indicating that the response obtained in older infants can be variable and can be partially predicted by age at OA treatment and baseline CHOP-INTEND scores. These findings will be of help when counselling families who may opt to switch therapy hoping to see the same magnitude of responses observed in infants treated at an earlier age.
Despite our study includes all the tertiary referrals for SMA in Italy, when the cohort is subdivided according to age, weight, number of copies etc., the numbers are still too small to draw any definitive conclusion or to provide suggestions. The number of variables to be considered will become even bigger when including different SMA types or different copy numbers that could not be included in our study. Larger international studies are therefore needed to merge findings from different countries and provide a larger dataset that will also allow to apply new methods of analysis, such as machine learning models that could help to identify trajectories of progression in individual subgroups.
Contributors
MP, BB, and AC equally contributed as first authors of the manuscript and performed material preparation, data collection, and analysis. EM, MP, BB, AC, and GC wrote the first draft of the manuscript. All authors contributed to the study conception and design and commented on previous version of the manuscript. All authors read and approved the final manuscript.
Data sharing statement
Individual data cannot be made openly available, in compliance with the signed informed consent. Aggregate data and study protocol will be available on request after the publication of the study.
Declaration of interests
No author has received financial support for the present manuscript. MP, GC, SM, RM, CP reported personal fees from Biogen, Novartis and Roche outside the submitted work; BB reported personal fees from Biogen and Novartis outside the submitted work; AdA reported personal fees from AveXis outside the submitted work; FR reported personal fees from Biogen outside the submitted work; DG, DL, CD, CT reported personal fees from Novartis outside the submitted work; MF reported personal fees from Sanofi and Amicus outside the submitted work; SC reported personal fees from Novartis and Scholar Rock outside the submitted work; RDS reported personal fees from Biogen and Roche outside the submitted work; RF reported personal fees from AveXis, Biogen, Capricor, families of SMA, Ionis Pharmaceuticals, Novartis, Roche, and ScholarRock outside the submitted work; EM reported personal fees from Biogen, Roche, Novartis, Scholar Rock, Epirium and Cytochinetics outside the submitted work; AC, AV, VAS, CA, CB, AP, RO, MR, IB, MS, CD, EA, CT, NB, EB have nothing to disclose.
Acknowledgments
EM is funded by grant from the Italian Ministry of Health (RF-2019-12370334). MCP is funded by grant from the Italian Ministry of Health (GR-2018-12365706).
ITASMAc group: Maria Carmela Pera, Chiara Bravetti, Marco Piastra, Orazio Genovese, Gianpaolo Cicala, Nicola Forcina, Sara Carnicella, Giulia Stanca, Michele Sacchini, Michela Catteruccia, Michele Tosi, Renato Cutrera, Claudio Cherchi, Maria Beatrice Chiarini, Francesca Salmin, Marina Pedemonte, Alessandra Govoni, Irene Mizzoni, Simone Morando, Riccardo Zanin, Enrica Rolle, Eleonora Salomon, Melania Giannotta, Gaia Scarpini, Antonio Toscano, Eloisa Gitto, Roberto Materia, Rossella D’Alessandro.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101997.
Contributor Information
Eugenio Mercuri, Email: eugeniomaria.mercuri@unicatt.it.
ITASMAc group:
Maria Carmela Pera, Chiara Bravetti, Marco Piastra, Orazio Genovese, Gianpaolo Cicala, Nicola Forcina, Sara Carnicella, Giulia Stanca, Michele Sacchini, Michela Catteruccia, Michele Tosi, Renato Cutrera, Claudio Cherchi, Maria Beatrice Chiarini, Francesca Salmin, Marina Pedemonte, Alessandra Govoni, Irene Mizzoni, Simone Morando, Riccardo Zanin, Enrica Rolle, Eleonora Salomon, Melania Giannotta, Gaia Scarpini, Antonio Toscano, Eloisa Gitto, Roberto Materia, and Rossella D’Alessandro
Appendix A. Supplementary data
References
- 1.Mercuri E., Bertini E., Iannaccone S.T. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11(5):443–452. doi: 10.1016/S1474-4422(12)70061-3. [DOI] [PubMed] [Google Scholar]
- 2.Kolb S.J., Coffey C.S., Yankey J.W., et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883–891. doi: 10.1002/ana.25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel R.S., McDermott M.P., Kaufmann P., et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercuri E., Sumner C.J., Muntoni F., Darras B.T., Finkel R.S. Spinal muscular atrophy. Nat Rev Dis Primers. 2022;8(1):52. doi: 10.1038/s41572-022-00380-8. [DOI] [PubMed] [Google Scholar]
- 5.Mendell J.R., Al-Zaidy S., Shell R., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 6.Day J.W., Finkel R.S., Chiriboga C.A., et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 7.Mercuri E., Muntoni F., Baranello G., et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(10):832–841. doi: 10.1016/S1474-4422(21)00251-9. [DOI] [PubMed] [Google Scholar]
- 8.McMillan H.J., Proud C.M., Farrar M.A., Alexander I.E., Muntoni F., Servais L. Onasemnogene abeparvovec for the treatment of spinal muscular atrophy. Expert Opin Biol Ther. 2022;22(9):1075–1090. doi: 10.1080/14712598.2022.2066471. [DOI] [PubMed] [Google Scholar]
- 9.Mendell J.R., Al-Zaidy S.A., Lehman K.J., et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78(7):834–841. doi: 10.1001/jamaneurol.2021.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Silva A.M., Holland S., Kariyawasam D., et al. Onasemnogene abeparvovec in spinal muscular atrophy: an Australian experience of safety and efficacy. Ann Clin Transl Neurol. 2022;9(3):339–350. doi: 10.1002/acn3.51519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S., Lee Y.J., Kong J., et al. Short-term clinical outcomes of onasemnogene abeparvovec treatment for spinal muscular atrophy. Brain Dev. 2022;44(4):287–293. doi: 10.1016/j.braindev.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Weiss C., Ziegler A., Becker L.L., et al. Gene replacement therapy with onasemnogene abeparvovec in children with spinal muscular atrophy aged 24 months or younger and bodyweight up to 15 kg: an observational cohort study. Lancet Child Adolesc Health. 2022;6(1):17–27. doi: 10.1016/S2352-4642(21)00287-X. [DOI] [PubMed] [Google Scholar]
- 13.Mercuri E., Finkel R., Scoto M., et al. Development of an academic disease registry for spinal muscular atrophy. Neuromuscul Disord. 2019;29(10):794–799. doi: 10.1016/j.nmd.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Glanzman A.M., Mazzone E.S., Young S.D., et al. Evaluator training and reliability for SMA global nusinersen trials 1. J Neuromuscul Dis. 2018;5(2):159–166. doi: 10.3233/JND-180301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord. 1995;5(1):3–5. doi: 10.1016/0960-8966(94)00075-k. [DOI] [PubMed] [Google Scholar]
- 16.Nelson W.E. Edition 21. Elsevier Inc; Philadelphia, PA: 2020. Nelson textbook of pediatrics. [Google Scholar]
- 17.Strauss K.A., Farrar M.A., Muntoni F., et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: the phase III SPR1NT trial. Nat Med. 2022;28(7):1390–1397. doi: 10.1038/s41591-022-01867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss K.A., Farrar M.A., Muntoni F., et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the phase III SPR1NT trial. Nat Med. 2022;28(7):1381–1389. doi: 10.1038/s41591-022-01866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chand D.H., Mitchell S., Sun R., LaMarca N., Reyna S.P., Sutter T. Safety of onasemnogene abeparvovec for patients with spinal muscular atrophy 8.5 kg or heavier in a global managed access program. Pediatr Neurol. 2022;132:27–32. doi: 10.1016/j.pediatrneurol.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Chand D., Mohr F., McMillan H., et al. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J Hepatol. 2021;74(3):560–566. doi: 10.1016/j.jhep.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Tosi M., Catteruccia M., Cherchi C., Mizzoni I., D'Amico A. Switching therapies: safety profile of onasemnogene abeparvovec-xioi in a SMA1 patient previously treated with risdiplam. Acta Myol. 2022;41(3):117–120. doi: 10.36185/2532-1900-077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual-Morena C., Cavero-Redondo I., Lucerón-Lucas-Torres M., Martínez-García I., Rodríguez-Gutiérrez E., Martínez-Vizcaíno V. Onasemnogene abeparvovec in type 1 spinal muscular atrophy: a systematic review and meta-analysis. Hum Gene Ther. 2022;34:129–138. doi: 10.1089/hum.2022.161. [DOI] [PubMed] [Google Scholar]
- 23.Ferrante L., Melendez-Zaidi A., Lindsey W., Lotze T. Novel use of nusinersen as a therapeutic bridge to onasemnogene abeparvovec-xioi in a premature neonate with type 1 spinal muscular atrophy. Muscle Nerve. 2022;66(2):E8–E10. doi: 10.1002/mus.27648. [DOI] [PubMed] [Google Scholar]
- 24.Mirea A., Shelby E.S., Axente M., et al. Combination therapy with nusinersen and onasemnogene abeparvovec-xioi in spinal muscular atrophy type I. J Clin Med. 2021;10(23):5540. doi: 10.3390/jcm10235540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oechsel K.F., Cartwright M.S. Combination therapy with onasemnogene and risdiplam in spinal muscular atrophy type 1. Muscle Nerve. 2021;64(4):487–490. doi: 10.1002/mus.27375. [DOI] [PubMed] [Google Scholar]
- 26.Harada Y., Rao V.K., Arya K., et al. Combination molecular therapies for type 1 spinal muscular atrophy. Muscle Nerve. 2020;62(4):550–554. doi: 10.1002/mus.27034. [DOI] [PubMed] [Google Scholar]
- 27.Chand D.H., Zaidman C., Arya K., et al. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J Pediatr. 2021;231:265–268. doi: 10.1016/j.jpeds.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 28.Yazaki K., Sakuma S., Hikita N., Fujimaru R., Hamazaki T. Child neurology: pathologically confirmed thrombotic microangiopathy caused by onasemnogene abeparvovec treatment for SMA. Neurology. 2022;98(19):808–813. doi: 10.1212/WNL.0000000000200676. [DOI] [PubMed] [Google Scholar]
- 29.Matesanz S.E., Battista V., Flickinger J., Jones J.N., Kichula E.A. Clinical experience with gene therapy in older patients with spinal muscular atrophy. Pediatr Neurol. 2021;118:1–5. doi: 10.1016/j.pediatrneurol.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Ali H.G., Ibrahim K., Elsaid M.F., et al. Gene therapy for spinal muscular atrophy: the Qatari experience. Gene Ther. 2021;28(10–11):676–680. doi: 10.1038/s41434-021-00273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.