Fig. 3 |. Nitrogen upshift drives glycolysis forward.
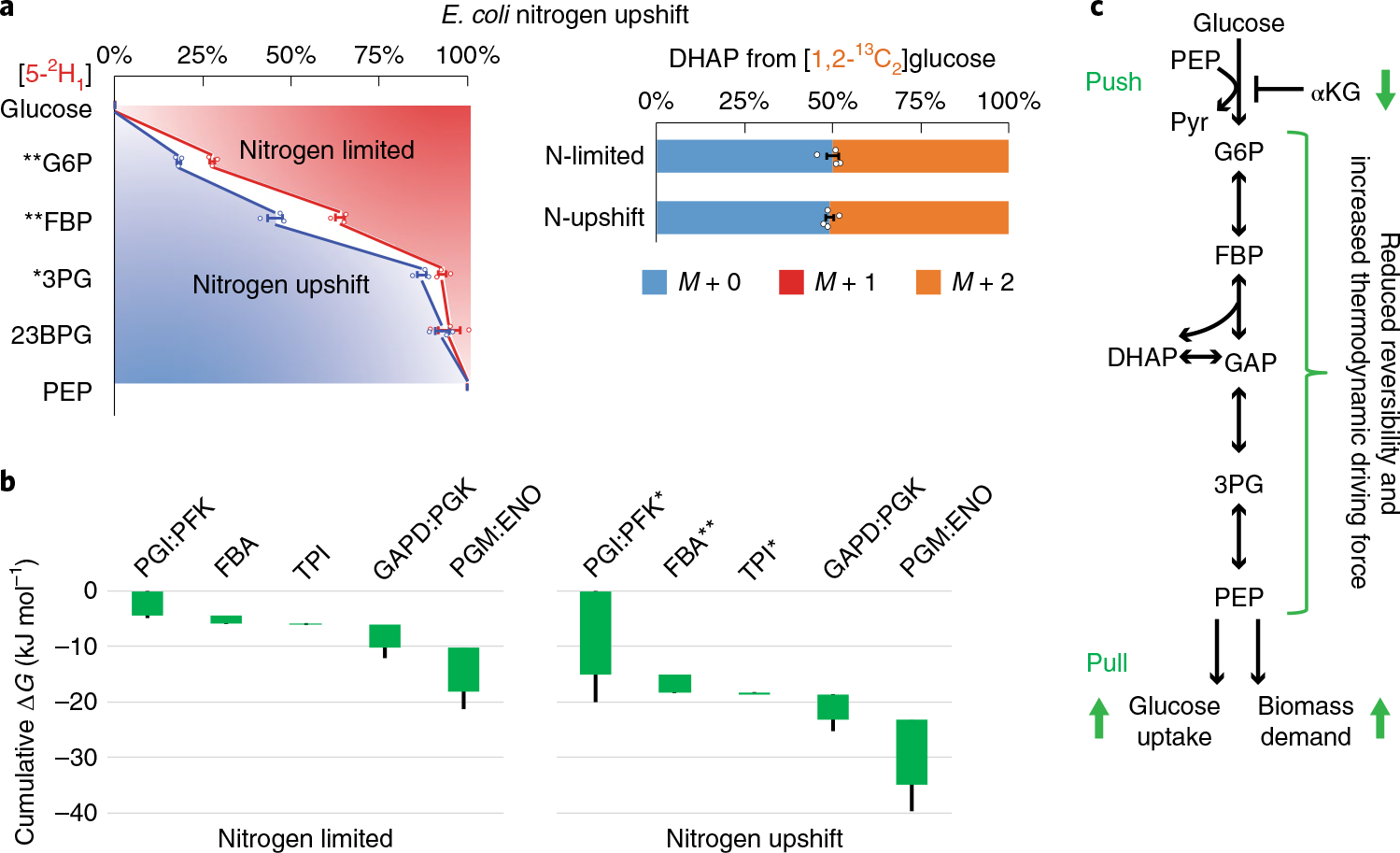
a, Isotope tracing of glycolytic reversibility in E. coli during nitrogen limitation and immediately following nitrogen upshift. E. coli were cultured on [5-2H1]- or [1,2-13C2]glucose with arginine as the sole nitrogen source, which supports slow cell growth (that is, nitrogen limitation). Glycolytic intermediates retained significantly less deuterium than in the NH4Cl fast growth condition, indicating greater glycolytic reversibility. With [1,2-13C2]glucose, DHAP was ~50% unlabeled, indicating high TPI reversibility. Five minutes after spiking in NH4Cl (that is, nitrogen upshift), most glycolytic intermediates gained substantial deuterium labeling, while the DHAP labeling did not change. The center and error bars represent the mean ± s.e.m. (n = 3 or 4, biologically independent samples). b, Corresponding ΔG were inferred from the forward and backward fluxes that best simulated the observed 2H and 13C labeling. Each of the reaction(s) ΔG (<0) is represented by the height of the green bar. The bottom edges indicate the cumulative ΔG up to the corresponding step in glycolysis. Whiskers show s.e.m. (Methods). On nitrogen upshift, glycolysis shifted forward, with the most drastic free energy drops occurring near the beginning and the end of glycolysis. c, Schematic of glycolytic flux regulation during nitrogen upshift. Enzyme I is activated by the drop of αKG, accelerating G6P production and PEP consumption, with the middle of the pathway responding via decreased reversibility. *P < 0.05 and **P < 0.01 by two-tailed t-tests or bootstrapping (Methods).
