Fig. 6 |. Slow glycolysis of C. cellulolyticum operates near equilibrium using PPi-dependent PFK.
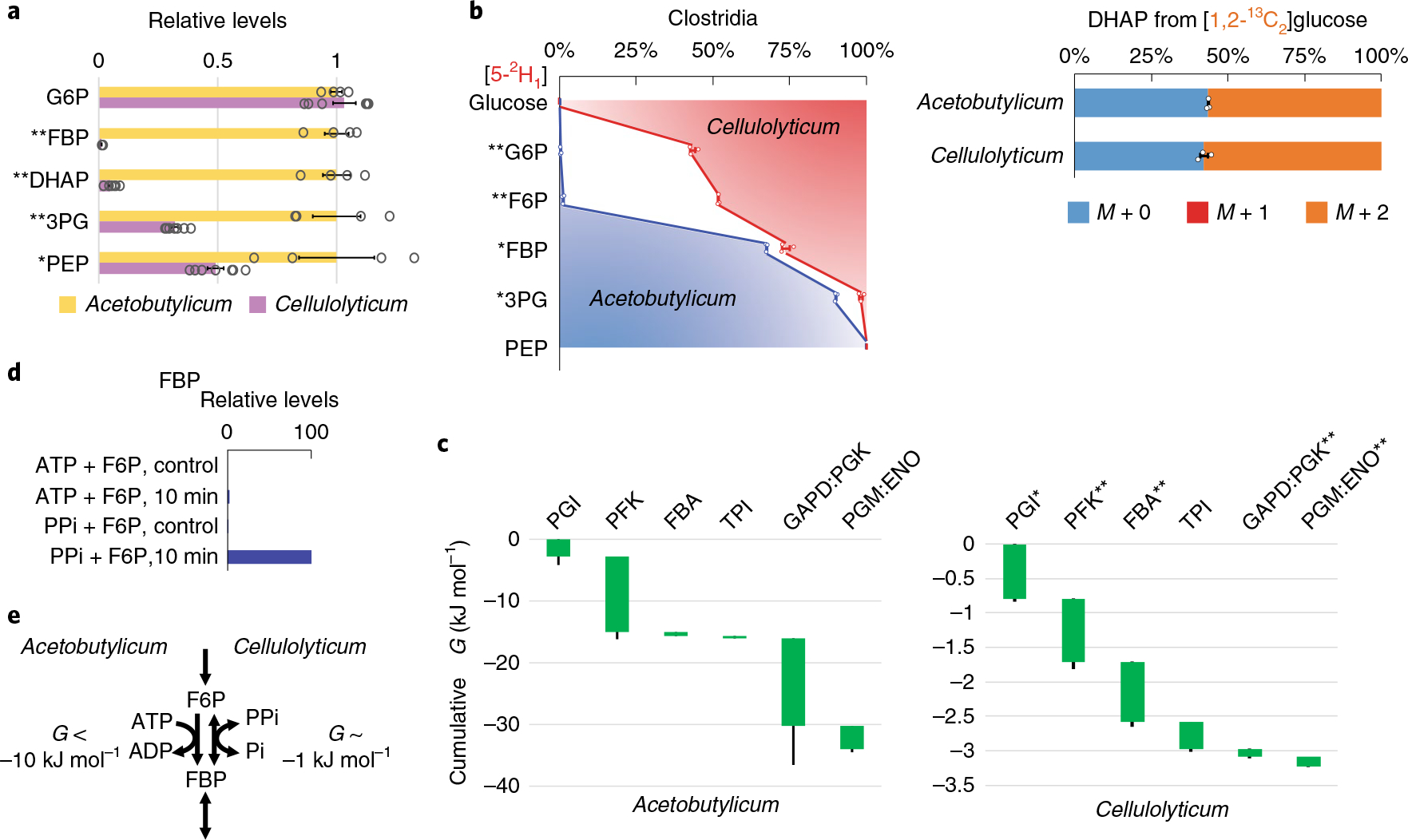
a, Relative levels of glycolytic intermediates in C. acetobutylicum versus C. cellulolyticum. Measurements are normalized by the means of the individual metabolites in C. acetobutylicum. The center and error bars represent the mean ± s.e.m. (n = 4 or 7, biologically independent samples). b, Isotope tracing of glycolytic reversibility in the obligate anaerobes C. cellulolyticum and C. acetobutylicum cultured on [5-2H1]- or [1,2-13C2]glucose. The center and error bars represent the mean ± s.e.m. (n = 3, biologically independent samples). c, All glycolytic reactions of C. cellulolyticum were close to equilibrium with ΔG > −1 kJ mol−1. The resulting cumulative ΔG from G6P to PEP was approximately −3 kJ mol−1, one-tenth of that of C. acetobutylicum. Each of the reaction(s) ΔG (<0) is represented by the height of the green bar. The bottom edges indicate the cumulative ΔG up to the corresponding step in glycolysis. Whiskers show s.e.m. (Methods). The most substantial differences between canonical (for example, E. coli, C. acetobutylicum and mammalian) and C. cellulolyticum glycolysis were in the ΔG of phosphofructokinase (PFK) and GAPD:PGK. d, In C. cellulolyticum cell lysate, fructose-1,6-bisphosphate (FBP) was produced in the presence of PPi but not ATP (10 min incubation). Control represents identical assays without cell lysate and the plotted results represent two replicate experiments. e, The weakly forward-driven PFK in C. cellulolyticum is due to the use of the PPi–Pi pair instead of the ATP–ADP pair. *P < 0.05 and **P < 0.01 by two-tailed t-tests or bootstrapping (Methods).
