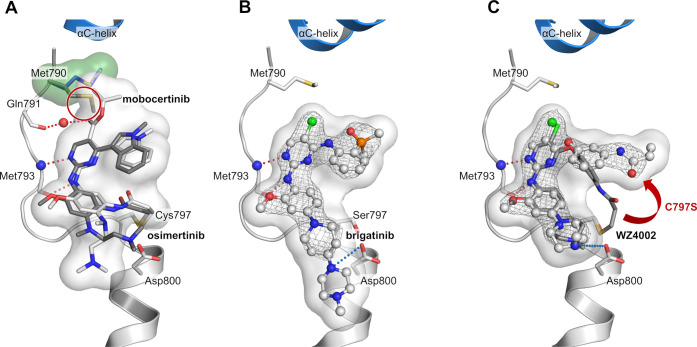
Figure 1.
Cocrystal structures of EGFR with different inhibitors were used for a structure-guided design approach of reversible aminopyrimidine-based inhibitors. (A) Comparison of osimertinib (gray, PDB 6JX4) and mobocertinib (white, PDB 7A6K) bound to EGFR-T790M. Hydrogen bond network of mobocertinib is in red, and the reorientation of Met790 (green surface) by the isopropyl ester is highlighted (red circle). (B) The ALK inhibitor brigatinib bound to EGFR-T790M/C797S (PDB 7ZYM). Ionic interactions of the front pocket substituent with Asp800 highlighted in blue. (C) WZ4002 (white) bound to EGFR-T790M/C797S (PDB 7ZYN) in comparison to WZ4002 (gray) bound to EGFR-T790M (PDB 3IKA) showing an additional ionic interaction with Asp800. The unfavorable reorientation of the acrylamide is highlighted (red arrow). All |2Fo–Fc| maps are contoured at a rmsd of 1. Simulated annealing |FoFc| omit electron density maps are shown in SI, Figure S1.